Burning logs—an age-old practice that many of us have experienced during a cozy evening by the fireplace or while camping under the starlit sky. But the elemental question arises: when we ignite these logs, are we truly conserving energy and matter, or are we, quite literally, setting them ablaze in a destructive dance? This inquiry poses a duality of matter and energy—the very fabric of our physical universe. Are they simply transformed, or do they cease to exist in their original forms?
To understand the nuances of energy and matter conservation during the combustion of logs, one must first delve into the principles governing these phenomena. The law of conservation of mass states that matter can neither be created nor destroyed in an isolated system. What occurs during the burning of logs is not the annihilation of matter but rather a transformation into different states. When a log is subjected to high temperatures, the complex organic molecules that compose it (primarily cellulose, hemicellulose, and lignin) break down. The combustion process initiates a series of chemical reactions, fundamentally altering the structure of these molecules.
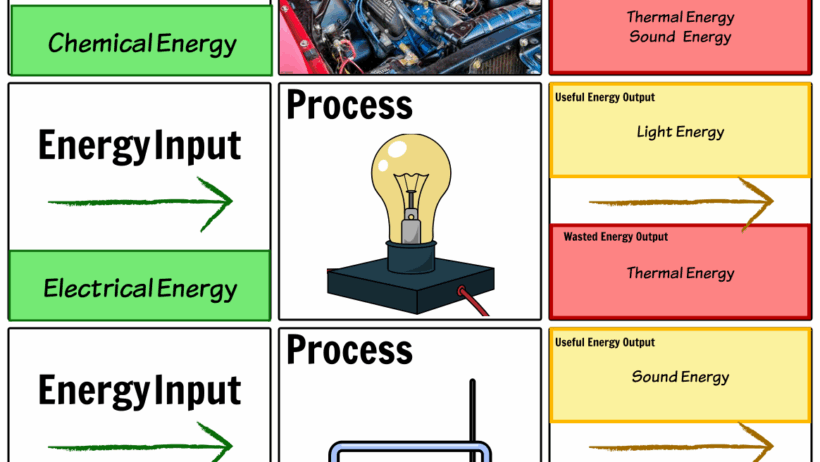
Moreover, let’s explore the combustion reaction—the chemical process that takes place when logs burn. This reaction primarily involves carbon, hydrogen, and oxygen. When logs ignite, they react with oxygen in the air, producing carbon dioxide and water vapor while releasing energy in the form of heat and light. This transformation is a quintessential example of energy conversion rather than destruction. The stored chemical energy in the bonds of the wood is liberated and transformed into thermal energy, illuminating our surroundings and warming our bodies. While matter undergoes a change, it doesn’t disappear; it simply metamorphoses into new substances. These byproducts—ash, gases, and heat—remain in existence but are manifested differently.
Energy, in a similar vein, follows the first law of thermodynamics: it cannot be created or destroyed; it can only change forms. When logs burn, the potential energy they possess is released as kinetic energy—heat and light. This phenomenon beckons a playful question: If the logs seem to vanish from our sight as they are consumed, where does that energy go? Think of it as a magical transformation—like a caterpillar emerging as a butterfly—where the energy from the logs is not lost but transformed into other, more useful forms. The unseen energy that heats our homes during winter or fuels our cooking is a testament to this energetic metamorphosis.
However, this process is not without ecological implications. The interplay of energy and matter during combustion is not solely a matter of chemistry; it heralds challenges in how we utilize and conserve our natural resources. Burning logs releases not just carbon dioxide, a greenhouse gas, but also particulate matter, which can have deleterious effects on air quality and human health. Thus, while energy may be conserved and transformed, the consequences of our choices lead to environmental concerns regarding air pollution and climate change.
The question then becomes, what role do we play in this cycle of energy transformation? As stewards of the environment, it is incumbent upon us to consider sustainable practices. Rather than relying solely on burning logs as a means of energy, we can explore alternatives—solar panels, wind turbines, and other renewable resources that align with the principles of conservation while mitigating detrimental impacts on our planet. Igniting logs may provide immediate benefits, but it is an ephemeral solution with long-term ramifications.
Furthermore, the efficiency of combustion must also be scrutinized. Not all logs burn equally—seasoned hardwoods yield a higher energy output compared to green or softwoods. The moisture content of the wood plays a pivotal role in energy conservation. Higher moisture levels can lead to incomplete combustion, wasting energy and releasing more pollutants into the atmosphere. This brings to light another consideration in our relationship with energy derived from natural resources: knowledge is paramount. Understanding what we burn and how we burn it is integral to reducing our ecological footprint.
Moreover, if we shift our perspective on logs from mere fuel sources to valuable components of an ecological system, we can become more conscientious consumers of energy. Trees play a vital role in carbon sequestration—the process where carbon dioxide is absorbed from the atmosphere, helping to mitigate climate change. Therefore, if we over-rely on burning logs without sustainable practices, we risk diminishing this important natural resource. Instead, opting for responsibly sourced firewood or engaging in forest stewardship can help ensure that we are not just extraction-based consumers but active participants in energy conservation.
To conclude, while the act of burning logs seems straightforward, it is steeped in complex interactions of energy and matter. Through the lens of conservation, we see that energy is not destroyed; it is transformed, while matter changes states but does not vanish. This inquiry into the nature of energy and matter around burning logs prompts us to reflect upon our energy choices and their wider ecological impacts. As we navigate the challenges posed by climate change, embracing sustainable options for energy usage will indeed reflect our commitment to conservation, ensuring the delicate balance of our environment is preserved for future generations.