Explosions are a captivating phenomenon, often evoking a mix of fascination and trepidation. The sheer force with which they occur can leave observers in awe, yet there’s a piquant question lurking beneath the pyrotechnic display: Do explosions conserve kinetic energy? To appreciate this complex query, one must delve into the intricacies of energy conservation, the transformations that occur during an explosion, and the resultant distribution of kinetic energy and heat.
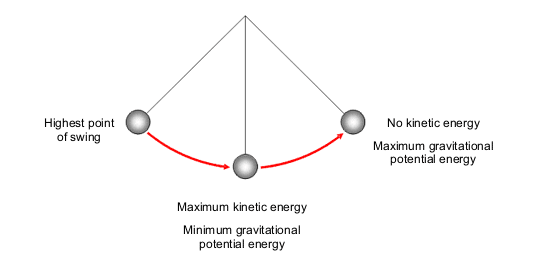
At its core, the principle of energy conservation states that energy cannot be created or destroyed; it can only be transformed from one form to another. This fundamental concept diverges into various domains of physics, providing insight into how energy behaves during vigorous events like explosions. An explosion typically involves a rapid release of energy, often through a chemical reaction leading to the formation of gases and heat. The striking transformation from potential energy—stored in the chemical bonds of molecules—to kinetic energy (the energy of motion) results in the dramatic outward burst characteristic of explosions.
When examining the mechanics of an explosion, it is paramount to consider what occurs during this cataclysmic shift. Initially, before the explosion, a substance—whether it be a fuel, explosive compound, or gas—contains a substantial amount of stored potential energy. Upon initiation, this energy is liberated explosively, propelling matter outward at high velocities. However, the kinetic energy produced does not emerge in isolation but rather in tandem with other forms of energy, primarily thermal energy and sound energy.
As the explosion unfolds, some of this energy is converted into kinetic energy of the resulting fragments. The rapid fragmentation results in a distribution of mass and velocity, which can indeed be analyzed to quantify the kinetic energy generated. However, an observable discrepancy arises: not all the potential energy transforms into kinetic energy. A significant portion is dissipated as heat due to friction, thermal expansion, and subsequent gas formation, which contributes to an increase in temperature of the surrounding environment.
It is essential to clarify how kinetic energy is distributed after an explosion. Imagine a grenade detonating in midair; it shatters into numerous small pieces propelled in various directions. Each fragment carries kinetic energy, calculated as ½ mv², where m stands for mass and v for velocity. The collective kinetic energy of these fragments may appear formidable, yet, when juxtaposed against the initial potential energy latent within the explosive material, one finds that a substantial portion is lost to non-kinetic energy forms. This observation leads to a pivotal realization: while kinetic energy is indeed generated during explosions, it does not fully account for the initial potential energy available.
Moreover, the conservation of kinetic energy in explosions is often contextual and contingent upon the system in question. In an ideal, closed system, where no energy dissipates to external factors (an almost impossible scenario), one might postulate that an ideal explosion could conserve kinetic energy. However, in practical applications, explosions engage with their surroundings, absorbing and redistributing energy in multifaceted forms. In essence, kinetic energy emerges, but the conservation principle in these chaotic events is nuanced, heavily influenced by heat production and reactive dynamics.
Another intriguing aspect of explosions focuses on the production of sound. The sonic boom that often accompanies an explosion is a byproduct of kinetic energy converting into sound waves as the shock front travels through the air. This transformation showcases yet another dimension of energy conversion: kinetic energy is transmuted into acoustic energy, which further exemplifies the complex interplay between different energy types during an explosive event.
From a broader perspective, one cannot overlook the implications of energy conservation and transformation in the realm of environmental science. Understanding the nuances behind explosions yields insights into energy efficiency, resource management, and potential applications in sustainable engineering. For instance, the design of controlled explosions can facilitate the mining of minerals with minimal energy loss or contribute to innovative energy systems that harness the byproducts of explosive reactions.
In conclusion, while explosions indeed produce kinetic energy, and a portion of the potential energy is transformed into this kinetic form, it is crucial to recognize that many factors—namely, heat, sound, and environmental interaction—mitigate perfect conservation. An explosion serves as a potent illustration of how energy operates under real-world conditions, marrying the rigorous laws of physics with the chaotic dance of energy transformation. This multifaceted perspective not only deepens our understanding of physical principles but also exemplifies the complexities inherent in studying energy dynamics in explosive contexts.