Entropy, in a thermodynamic context, is often characterized as a measure of disorder or randomness in a system. It has significant implications in understanding the dynamics of energy within physical systems. One might whimsically ponder: if entropy is an inherent aspect of thermodynamic systems, does it, then, follow the law of energy conservation? This question invites an exploration into the relationship between entropy and energy considerations, revealing the subtle nuances that lie within the laws of thermodynamics.
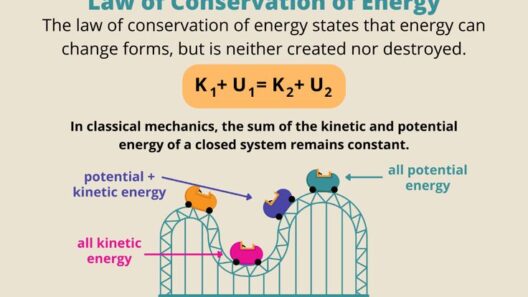
The law of energy conservation, also known as the first law of thermodynamics, states that energy cannot be created or destroyed in an isolated system. Instead, energy can be transformed from one form to another, such as from kinetic energy to potential energy or from chemical energy to thermal energy. This foundational principle establishes a systematic framework for analyzing energy transactions in various processes. However, while conserving energy is paramount in these transformations, entropy introduces complexity into the understanding of energy’s role in nature.
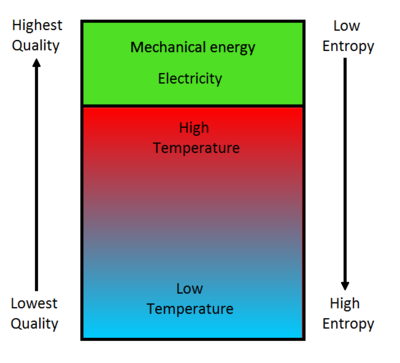
In line with the second law of thermodynamics, entropy tends to increase in an isolated system over time, leading to a directionality in energy dispersal known as the arrow of time. Imagine a newly built, aesthetically pleasing sandcastle. Initially, it exhibits minimal entropy, presenting order and structure. Over time, natural elements—wind, water, and human interference—erode its form, increasing disorder within the system, which brings to light a crucial idea: while the total amount of energy in the system remains constant, the distribution of that energy—and thus the arrangement of matter—plays a vital role in the evolution of that system.
This relationship between energy dispersion and entropy can pose an intriguing challenge: does ensuring energy efficiency equate to a decrease in entropy? To tackle this inquiry, one must consider that increasing efficiency typically implies a reduction in energy wastage and an enhancement in useful work output. Nevertheless, achieving greater energy efficiency may, in some instances, paradoxically lead to increased entropy within the surroundings. For example, when a power plant operates more efficiently, it may still release waste heat into the environment, contributing to an overall increase in entropy beyond the immediate closed system.
This interplay invites contemplation on the distinctions between local and global entropy. While a local decrease in entropy can occur during certain processes—such as the formation of complex structures in biological systems—the overarching trend remains that the total entropy of the universe is always on an upward trajectory. This phenomenon is aptly illustrated through various natural processes. Leaf formation during photosynthesis, for instance, represents a local decrease in entropy. Yet, when factoring in the energy expended by the sun and the subsequent release of heat into the atmosphere, the net effect remains an increase in the universe’s overall entropy.
Furthermore, the implications of entropy stretch far beyond the confines of thermodynamics. In ecological terms, systems often operate under constraints that reflect both energy conservation and entropy principles. Consider a food web in a natural environment. Energy flows from primary producers to apex predators; yet, at each trophic level, inefficiencies arise that result in energy dissipation, primarily as heat, thereby increasing the entropy of the system. This leads to a crucial observation: while energy in the form of food sustains life, the conversion processes invariably contribute to the entropy of the ecosystem as a whole.
Additionally, the concept of entropy extends its reach into discussions surrounding climate change. The relentless increase in entropy can manifest in various detrimental environmental changes. As fossil fuels combust, for example, they generate both energy for human purposes and an increase in entropy through the release of greenhouse gases. This unintended consequence supercharges climate systems, leading to unpredictable weather patterns and extreme environmental shifts. Such effects underscore the critical need for sustainable energy practices that minimize entropy’s untamed consequences on the planet.
Thus, unraveling whether entropy follows the law of energy conservation leads us to an intricate tapestry woven from the threads of thermodynamics, ecology, and environmental stewardship. Energy is indeed conserved, but its transformation bears a deeper relationship with entropy. Energy systems are governed by both laws, yet the interplay between them reveals complexity rather than simplicity.
In conclusion, while the law of energy conservation remains steadfast, entropy’s omnipresence serves as a reminder of the balance between order and disorder. It challenges us to navigate the energy landscape with a mindful approach, embracing innovations in efficiency while recognizing that our endeavors may sometimes paradoxically amplify the complexities of entropy in our environments. The challenge lies not only in conserving energy but also in understanding the broader implications of our actions on entropy—a nuanced consideration in the pursuit of a sustainable future.