Mass and energy, two fundamental quantities in physics, intertwine in a profound manner that influences the conservation of energy, a foundational principle across various scientific disciplines. This article delves into the relationship between mass and the conservation of energy, exploring various scenarios and implications for our understanding of the universe.
The conservation of energy principle asserts that the total energy in a closed system remains constant, although it may transform from one form to another. The inquiry into whether mass impacts this equation necessitates an exploration of different forms of energy, including kinetic, potential, thermal, and even the enigmatic realm of nuclear energy. To understand energy transformation and conservation, one must first comprehend the concept of mass. Mass is often perceived merely as a measure of matter in an object, but in the context of physics and energy conservation, its implications are much deeper.
At the most rudimentary level, mass is inherently related to energy as elucidated in Einstein’s celebrated equation, E=mc². This iconic formula exemplifies the direct relationship between mass (m) and energy (E), with c representing the speed of light in a vacuum, a constant. This equation articulates that mass can be converted to energy and vice versa. Thus, in any interaction where mass is converted into energy—such as in nuclear reactions—the conservation of energy still holds true. The mass that is lost during such a reaction manifests as energy released, aligning perfectly with the conservation principle.
Furthermore, the kinetic energy of an object is intrinsically tied to its mass. The equation for kinetic energy, KE = ½mv², illustrates that an increase in mass leads to a proportional increase in kinetic energy, provided the velocity remains unchanged. This relationship underscores that, in mechanical systems, mass is a conduit for energy transfer. When two masses collide, kinetic energy transfers between them, resulting in transformations that adhere to conservation laws. Observations of collisions in particle physics, for instance, reveal the conservation of momentum and energy, reaffirming that mass remains a significant player in energy dynamics.
Potential energy is another critical aspect of energy transformation where mass plays an imperative role. The gravitational potential energy, defined as PE = mgh (where h is the height and g is the acceleration due to gravity), delivers insight into how mass influences energy in gravitational fields. When an object is raised, it accumulates gravitational potential energy proportional to its mass and height. If this object falls, the potential energy is converted into kinetic energy, again adhering to the conservation principles. This example serves to establish the non-negligible impact of mass on energy states within gravitational systems.
Thermal energy comprises another vital segment of energy conservation. When discussing the conservation of energy, one must consider how mass affects the thermal capacity of a substance. The thermal energy content of a material correlates with its mass, specifically through the specific heat capacity, which defines the amount of heat required to change the temperature of a unit mass of the material. Thus, in caloric contexts where energy transfer occurs via heating, mass dictates the extent of thermal energy alteration, underscoring that mass is pivotal in energy conservation scenarios within thermal dynamics.
Another fascinating dimension emerges when contemplating the relationship between mass and energy at a cosmological scale. In astrophysics, the interplay of mass and energy profoundly impacts the evolution of stellar bodies. During fusion processes in stars, mass diminishes while energy proliferates, radiating outward and impacting surrounding celestial mechanics. This conservation of energy translates to observable phenomena such as stellar luminosity and radiation. Therefore, the mass-energy equivalence underpins not just local systems but extends its influence to cosmic scales, linking the fate of galaxies and their energy outputs to fundamental mass considerations.
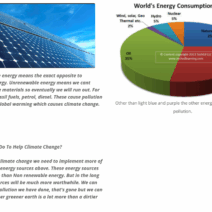
Notably, understanding the implications of mass on energy conservation extends beyond theoretical frameworks; it holds significant real-world applications. In the realm of environmental science, for instance, recognizing how energy consumption and mass correlate is pivotal in addressing climate change. Energy-intensive processes often correlate with substantial mass exchanges, particularly in manufacturing and energy generation. Hence, conservation strategies that consider mass reduction in processes—such as improving efficiency or transitioning to renewable energy sources—can have profound implications for lowering aggregate energy use and emissions.
Moreover, in the context of sustainable engineering and design, knowledge of mass and its influence on energy consumption allows for innovative solutions. From material selection in construction to optimizing transportation systems, reducing mass where appropriate can decrease energy demands, thus conserving energy and mitigating environmental impacts. For instance, lighter materials in vehicle manufacturing lead to reduced fuel consumption, exemplifying how critical mass considerations are in energy conservation efforts.
In conclusion, mass significantly impacts the conservation of energy equation across various contexts and phenomena. From the cosmic interplay of stars to the nuances of everyday energy consumption, the relationship between mass and energy is pivotal. Recognizing and addressing this relationship can lead to innovative solutions and strategies crucial in combating climate change and fostering a more sustainable future. Understanding these complex dynamics is imperative, as society seeks to balance energy demands while minimizing environmental impacts.