Cellular respiration is a fundamental biological process through which organisms convert nutrients into usable energy, specifically adenosine triphosphate (ATP). This intricate phenomenon not only underscores the interconnectedness of life but also serves as a compelling case study in the conservation of matter and energy. The interplay between these two principles is pivotal for sustaining life on Earth.
To appreciate the conservation of matter and energy during cellular respiration, one must first understand the overall equation governing this vital process. Cellular respiration can be simplified into a balanced chemical equation: glucose (C₆H₁₂O₆), in the presence of oxygen (O₂), is transformed into carbon dioxide (CO₂), water (H₂O), and energy (ATP). This reaction can be illustrated as follows:
C₆H₁₂O₆ + 6 O₂ → 6 CO₂ + 6 H₂O + energy (ATP)
Now, let us delve deeper into the key stages of cellular respiration, which encompass glycolysis, the Krebs cycle, and the electron transport chain (ETC). Each stage is a remarkable testament to the law of conservation of mass and energy.
Glycolysis is the initial phase of cellular respiration, occurring in the cytoplasm of the cell. Here, a single molecule of glucose undergoes a series of enzymatic reactions, ultimately resulting in the production of two pyruvate molecules, along with a modest yield of ATP and NADH. Remarkably, the number of carbon atoms is preserved throughout this transformation. The six carbon atoms in the glucose molecule reappear in the pyruvate molecules. Thus, matter conservation is visibly demonstrated at this early stage.
Following glycolysis, the pyruvate enters the mitochondria, where it undergoes decarboxylation – a crucial transition to the Krebs cycle. In this phase, the pyruvate is further oxidized, releasing CO₂ as a byproduct. The carbon atoms, once part of glucose, are now transformed into carbon dioxide, which is eventually expelled from the organism’s body, often via respiration. However, the fundamental question arises: How does this cyclical movement of carbon contribute to a greater understanding of environmental sustainability?
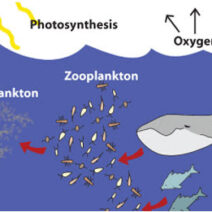
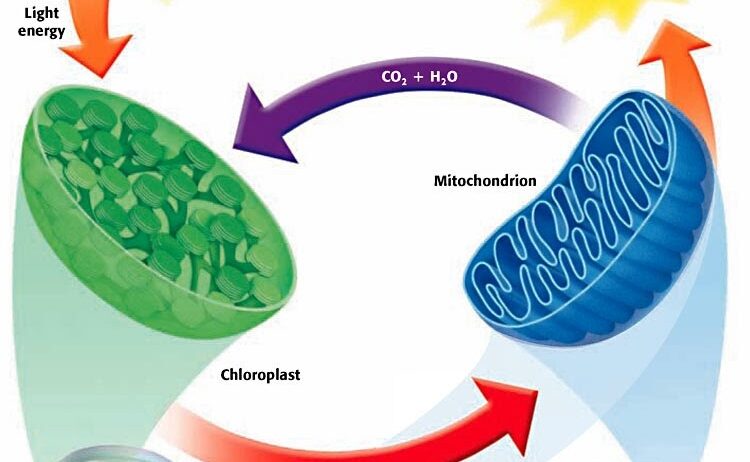
As carbon is released into the atmosphere, it re-enters the biogeochemical cycle, primarily through the process of photosynthesis in plants. This cyclical exchange underscores a breathtaking ecological balance wherein carbon is utilized by plants to generate glucose, thereby fostering a perpetual loop of matter conservation. The implications for climate change are significant; understanding this cycle fosters a greater appreciation for the role of vegetation in sequestering atmospheric CO₂, an essential aspect of mitigating global warming.
Continuing through the Krebs cycle, various intermediate compounds are also generated, such as NADH and FADH₂, which are pivotal as electron carriers. These reduced coenzymes are subsequently utilized in the electron transport chain (ETC), located within the inner mitochondrial membrane. The ETC is arguably the most awe-inspiring segment of cellular respiration, where the energy stored in NADH and FADH₂ is harnessed to produce a substantial amount of ATP.
During this process, electrons are transferred through a series of proteins, ultimately reducing molecular oxygen to form water. Notably, the conservation of energy is unequivocally illustrated here. The energy released from the electrons is not lost; it is meticulously captured in the form of a proton gradient across the inner mitochondrial membrane, driving ATP synthesis via ATP synthase. This transformation of potential energy into a usable form epitomizes the principle of energy conservation, as energy cannot be created or destroyed but only converted.
As the cycle continues, the remaining matter is accounted for through the metabolic processes that recycle intermediates and byproducts. For instance, during cellular respiration, some of the carbohydrates we consume are used for cellular repair and maintenance, ensuring a steady flow of carbon for various biosynthetic pathways. Additionally, the conservation of matter is reflected in various cellular activities, including the synthesis of macromolecules and nucleic acids, essential for life.
Another often-overlooked aspect of cellular respiration is the role of anaerobic processes in environments devoid of oxygen. While aerobic respiration is more efficient in energy yield, organisms can still conserve matter and energy through anaerobic fermentation pathways. In these scenarios, glucose is still metabolized, albeit with lower ATP yield and through alternative reactions, resulting in byproducts like lactic acid or ethanol, demonstrating adaptability and resource conservation in biological systems.
In conclusion, the conservation of matter and energy during cellular respiration is an integral and beautifully orchestrated process that sustains life on Earth. From the delicate balance of carbon cycling through ecosystems to the intricate mechanisms of ATP production, it undeniably illustrates the profound interconnectedness of biological systems. Observing the nuances of cellular respiration invites a deeper appreciation for the mechanisms underpinning life and underscores a critical understanding of how these processes relate to larger environmental issues, including climate change. Recognizing this significance can inspire a sense of responsibility towards the planet and its preservation for future generations.