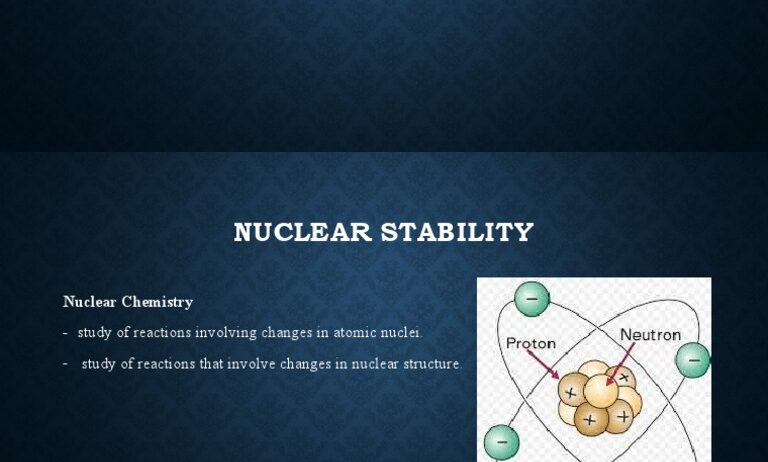
Nuclear reactions serve as profound illustrations of two fundamental principles in physics: the conservation of energy and the conservation of mass. Both principles provide a framework for understanding the intricate processes that govern the behavior of atomic nuclei. As society grapples with energy demands and environmental concerns, nuclear reactions offer insight into how energy can be harnessed while also posing challenges that must be addressed. The phenomena manifested by nuclear reactions ignite curiosity and foster a deeper understanding of the universe’s mechanics.
At the core of nuclear physics lies the concept of energy transformation. The first law of thermodynamics mandates that energy cannot be created or destroyed, only transformed from one form to another. In nuclear reactions, this principle is vividly displayed. For instance, during the fission process, heavy atomic nuclei such as Uranium-235 or Plutonium-239 split into lighter nuclei. This transformation releases a substantial amount of energy, owing primarily to a phenomenon known as mass-energy equivalence, articulated by Albert Einstein’s famous equation (E=mc^2). This equation indicates that mass (m) can be converted into energy (E) with c representing the speed of light in a vacuum, a constant that emphasizes the conversion ratio between mass and energy is extraordinarily large.
The immense energy released during fission is a result of the binding energy that holds protons and neutrons together within an atomic nucleus. When a nucleus undergoes fission, the products of the reaction—the lighter nuclei—have a higher binding energy per nucleon compared to the original heavy nucleus. In essence, the fission process reorganizes the nucleons into a more stable configuration, wherein the surplus energy is emitted, typically in the form of kinetic energy of the reaction products as well as radiation.
Conversely, the process of fusion, which occurs in stars, especially our Sun, is another striking example of nuclear reactions illustrating these conservation principles. In fusion, lighter nuclei, such as hydrogen isotopes, combine to form a heavier nucleus, helium. Similar to fission, the fusion process also leads to the liberation of energy, arising again from the difference in binding energy before and after the reaction. The fusion of hydrogen isotopes into helium releases energy that sustains stellar bodies and, by extension, provides solar energy essential for life on Earth.
The fascination with nuclear reactions is compounded by the profound implications they have for our understanding of both energy production and mass conservation. A curious observer may notice that despite the tangible transformations occurring within these reactions, the total mass-energy remains invariant. For example, in fission and fusion reactions alike, after accounting for the mass of all reactants and products, and considering their energy equivalents, the total will always align with the masses defined by (E=mc^2). This invariant quality nurtures a sense of wonder in the natural world’s consistency despite the chaos that nuclear reactions can inflict.
Moreover, the conservation of mass and energy presented through nuclear reactions prompts critical discussions surrounding energy sustainability and environmental impacts. As global energy consumption escalates, nuclear energy, derived from fission processes, emerges as a compelling alternative to fossil fuels. It boasts high energy density, meaning it can produce vast amounts of energy from relatively small amounts of fuel. This capacity aligns well with needs for reducing greenhouse gas emissions, thus addressing climate change concerns effectively. In particular, as nations seek to transition towards cleaner energy sources, the nuclear option may mitigate reliance on carbon-intensive resources.
However, the advantages of nuclear energy must be weighed against its disadvantages, which include radioactive waste management, potential catastrophic failures, and the high costs associated with establishing nuclear infrastructure. The interplay of these factors often elicits robust debate, underscoring the complexity of harnessing nuclear energy safely and ethically. Herein lies another layer of fascination: the balancing act between technological advancement and ecological stewardship. Society stands at a crucial juncture, grappling with the nuances of energy choice, environmental implications, and the overarching laws of nature.
The philosophical implications extend into the realm of ethics as well. Scientific advancements in nuclear technology raise questions about humanity’s right to wield such formidable power. As nuclear reactions succinctly illustrate the conservation of energy and mass, they simultaneously challenge humanity to ponder the responsibilities that accompany such knowledge. The moral implications of nuclear proliferation and warfare cannot be ignored. The destructive potential inherent in nuclear technology serves as a stark reminder of the power we hold.
In summary, nuclear reactions epitomize the principles of mass and energy conservation, offering profound insights into the workings of our universe. Whether through fission’s dramatics or fusion’s subtleties, the dynamic transformations that occur at the atomic level amplify our understanding of energy production. However, alongside this enlightenment comes a collection of challenges and responsibilities, urging society to tread carefully as we navigate our energy future. Ultimately, the fascination with nuclear reactions is not simply rooted in the science; it lies in their capacity to evoke reflection on human responsibility in the context of environmental sustainability and global well-being.