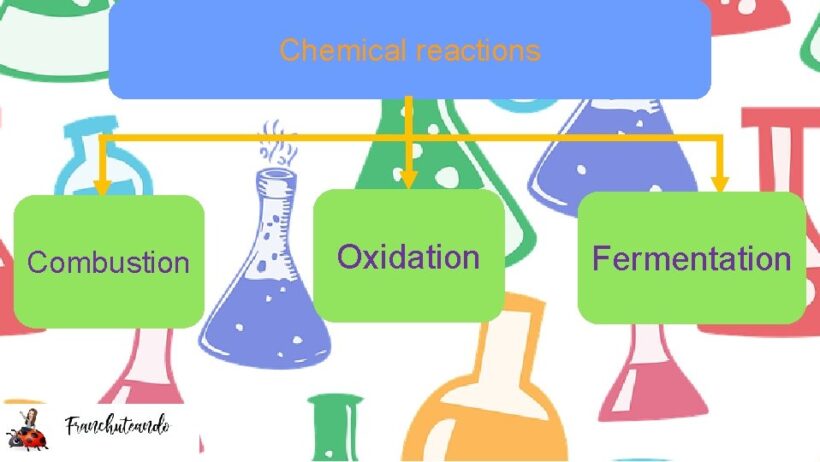
Energy conservation is an intriguing concept central to understanding chemical reactions, particularly in the contexts of combustion and fermentation. These processes demonstrate how energy is transformed rather than created or destroyed, aligning closely with the fundamental principle of thermodynamics. This discussion elucidates how energy conservation manifests in these reactions, revealing insights that prompt a reevaluation of conventional perspectives on energy usage and sustainability.
At its core, the law of conservation of energy asserts that energy can neither be created nor destroyed, only converted from one form to another. This principle becomes apparent in chemical reactions where bonds between atoms are rearranged. The energy stored in chemical bonds is released or absorbed depending on the reaction type, whether it be exothermic, like combustion, or endothermic, as seen in some fermentation processes.
Combustion is a quintessential example of an exothermic reaction. In this process, a substance, typically a hydrocarbon, reacts with oxygen to produce carbon dioxide, water, and heat. The efficiency of energy conservation during combustion lies in the idea that energy stored within chemical bonds is released in the form of heat and light. Consider the combustion of gasoline in an engine. The bonds between carbon and hydrogen atoms are broken, and the energy released from these bonds is harnessed to propel a vehicle. However, combustion systems are not impervious; they often yield emissions and heat loss, highlighting the need for better energy capture technologies.
In an environmentally conscious framework, it’s imperative to scrutinize the implications of combustion on climate change. The release of carbon dioxide as a byproduct contributes to the greenhouse effect. Thus, transitioning toward renewable energy sources, which utilize different chemical reactions with lower environmental impact, becomes paramount. Innovative approaches like biocombustion from biomass highlight how energy conservation can also align with environmental stewardship. Through careful management, we can retain energy within the system while minimizing carbon output.
Shifting focus, fermentation introduces a different paradigm of energy conservation. This biochemical process, prevalent in yeast and some bacteria, involves converting sugars into cellular energy through anaerobic respiration. Unlike combustion, fermentation is endothermic; it absorbs energy from its surroundings. The principal reaction involves breaking down glucose into ethanol and carbon dioxide, allowing organisms to derive energy in the absence of oxygen.
While it might initially appear that fermentation is less efficient in energy conservation, the process displays a different value chain. Fermentation yields energy that is stored in the form of ATP, adenosine triphosphate, which is essential for cellular functions. In this way, energy is conserved in a biological system, facilitating life processes even when atmospheric conditions are suboptimal for aerobic respiration. Moreover, fermentation plays a crucial role in sustainable energy solutions, prominently featured in biofuel production. Using organic waste to produce ethanol not only conserves energy but also addresses waste management issues—an exemplary model of circular economy principles.
A fascinating facet of energy conservation in chemical reactions is the role of catalysts. Catalysts are substances that increase the rate of a reaction without being consumed. In both combustion and fermentation, catalysts allow for more efficient energy transformations. For instance, metal catalysts can facilitate combustion in engines, improving fuel economy and reducing emissions. In fermentation, enzymes act as biological catalysts, streamlining chemical processes to maximize energy extraction from substrates.
As we navigate the complexities of energy conservation, it becomes increasingly evident that innovation is key. Investigating and refining chemical reactions can yield substantial benefits in energy efficiency. Emerging research areas, such as synthetic biology, explore engineered organisms capable of performing advanced fermentation processes with higher yield efficiencies, representing a frontier in energy conservation methods. Here, the synthesis of novel biomolecules could potentially revolutionize how we conserve and utilize energy sources.
Educational initiatives also play a crucial role in fostering awareness and understanding of energy conservation in chemical reactions. By exploring the mechanics of combustion and fermentation through practical applications, individuals can gain a tangible grasp of these processes. Schools and institutions should incorporate curriculum components that focus on sustainable practices. For instance, experiments involving fermentation can be coupled with discussions on its impact on energy conservation and waste reduction. This multifaceted approach encourages stewardship among young learners, cultivating a generation that prioritizes sustainability.
Moreover, the potential for conserving energy during chemical reactions invites us to reevaluate existing infrastructures. Manufacturing processes currently reliant on combustion could shift towards fermentation-based systems, reducing greenhouse gas emissions and conserving energy. Industries must adopt practices that embrace this transition, leveraging advancements in biotechnology and materials science to optimize energy use.
The broader implication of energy conservation during chemical reactions extends beyond individual practices to encompass global policies. Strategic shifts toward cleaner energy practices are essential for combating climate change. Governments and organizations should prioritize incentives for research in alternative reactions that minimize carbon emissions. Policies that encourage the adoption of cutting-edge technologies could reshape our energy portfolio, ensuring sustainability while meeting societal demands.
In conclusion, the intricacies of energy conservation during chemical reactions underscore a world ripe for exploration and innovation. By understanding the dynamics of processes such as combustion and fermentation, we can adopt a more informed approach in our fight against climate change. The potential for improvement is vast, as we stand at the precipice of a transformative shift towards sustainable energy utilization—one that harmonizes with the natural world and conserves our precious energetic resources for generations to come.