Cellular respiration is a fundamental biochemical process through which cells convert glucose into energy, enabling the myriad functions of life. This intricate process encapsulates a sophisticated interplay between catabolic and anabolic pathways, culminating in the production of adenosine triphosphate (ATP), the cellular energy currency. The understanding of how energy is conserved during the cellular respiration of glucose sheds light on the efficiency of biological systems and their role in maintaining life on Earth.
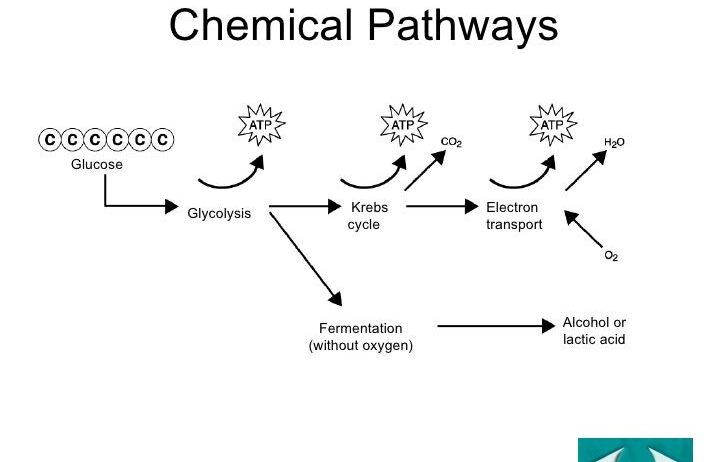
The process of cellular respiration can be broadly divided into three primary stages: glycolysis, the citric acid cycle (also known as the Krebs cycle), and oxidative phosphorylation. Each of these stages plays a critical role in the conversion of glucose, a six-carbon sugar, into usable energy while minimizing energy loss.
Initially, in the cytoplasm, glycolysis commences the breakdown of glucose into two molecules of pyruvate. This anaerobic process does not require oxygen and produces a net gain of two ATP molecules and two reduced NADH molecules. During glycolysis, glucose undergoes a series of enzymatic reactions, which ultimately facilitate its phosphorylation and cleavage into smaller molecules. The phosphorylation process, where phosphate groups are added to glucose, serves to destabilize the glucose molecule, making it more susceptible to enzymatic reactions. Importantly, while glycolysis generates a rudimentary amount of ATP, it also serves to harness critical reducing power in the form of NADH, which will be utilized in subsequent stages of respiration to maximize energy yield.
Following glycolysis, the pyruvate molecules, produced in the cytoplasm, enter the mitochondria where they are further oxidized. Before entering the citric acid cycle, each pyruvate molecule undergoes a decarboxylation process, resulting in the production of acetyl-CoA and the release of carbon dioxide (CO2). This step is crucial as it links glycolysis with the citric acid cycle and contributes to the conservation of energy by ensuring that acetyl-CoA can enter a highly productive series of reactions that extract further energy from carbon substrates.
The citric acid cycle, occurring within the mitochondrial matrix, represents a central hub for energy metabolism. Here, acetyl-CoA combines with oxaloacetate to form citrate, initiating a cyclic pathway characterized by a series of redox reactions. During this cycle, substrate-level phosphorylation occurs, leading to the production of ATP, as well as high-energy electron carriers NADH and FADH2. Each turn of the citric acid cycle generates three NADH, one FADH2, and one ATP (or GTP), highlighting the cycle’s efficiency in conserving energy from the degradation of glucose. Additionally, for each acetyl-CoA molecule processed, two molecules of CO2 are released, further emphasizing the need for glucose to be metabolized in an efficient and controlled manner to minimize energy wastage.
As glucose catabolism proceeds through glycolysis and the citric acid cycle, the pathway’s ultimate goal is to maximize the harvest of electrons, which will be transferred to the electron transport chain in the final stage of cellular respiration. Oxidative phosphorylation is where the majority of ATP is generated, and it is here that the conservation of energy reaches its zenith. The electron transport chain, located in the inner mitochondrial membrane, comprises a series of protein complexes that facilitate electron transfer through redox reactions. As NADH and FADH2 donate their electrons, a series of exergonic reactions occurs, leading to the pumping of protons (H+) across the membrane and creating a proton gradient.
This proton motive force is vital for ATP synthesis, as the protons flow back across the inner mitochondrial membrane through ATP synthase, a complex enzyme that harnesses the energy from the gradient to synthesize ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi). This mechanistic interplay ensures that a substantial yield of ATP is produced—approximately 30 to 32 molecules of ATP can be generated from one molecule of glucose, depending on the efficiency of the transport processes and the conditions within the mitochondria. This staggering conversion efficiency illustrates how cellular respiration is designed to maximize energy conservation, utilizing glucose as a primary fuel source while minimizing waste.
Furthermore, the orchestration of these metabolic pathways is controlled through intricate regulatory mechanisms, ensuring that energy conservation accompanies cellular demands. Enzymes involved in glycolysis, the citric acid cycle, and oxidative phosphorylation are subject to allosteric regulation, competitive inhibition, and feedback mechanisms that adapt to cellular energy requirements. For instance, high levels of ATP can inhibit glycolytic enzymes, signaling that energy production can be scaled back. Conversely, when energy stores are depleted, intermediates like ADP and AMP can serve as positive regulators, stimulating pathways that increase ATP production. This dynamic sensing system reinforces the efficiency of energy conservation in cellular respiration.
In conclusion, cellular respiration epitomizes the biological principle of energy conservation, leveraging the oxidation of glucose to synthesize ATP with remarkable efficiency. By dissecting the intricate stages of glycolysis, the citric acid cycle, and oxidative phosphorylation, one can appreciate the seamless transitions and interconnections that underpin this metabolic pathway. The conservation of energy not only fuels cellular functions but also orchestrates the broader symphony of life, underscoring the delicate balance between energy production and consumption that sustains ecosystems on our planet. Understanding these processes is paramount, especially in our current era of climate change, as it highlights the importance of energy efficiency in sustaining life while emphasizing the need for responsible resource management in an energy-dependent world.