Energy, much like the pulse of life coursing through all living beings, perpetually flows in chi-like currents that govern the intricate dance of chemical reactions within our universe. At the heart of these dances lie two fascinating categories: endothermic and exothermic reactions. Each of these paradigms represents not merely a transfer of energy but a profound narrative of exchange, transformation, and conservation. Understanding how energy is conserved in these processes sheds light on the fundamental principles that govern both chemistry and our environment.
Defining the Dynamics
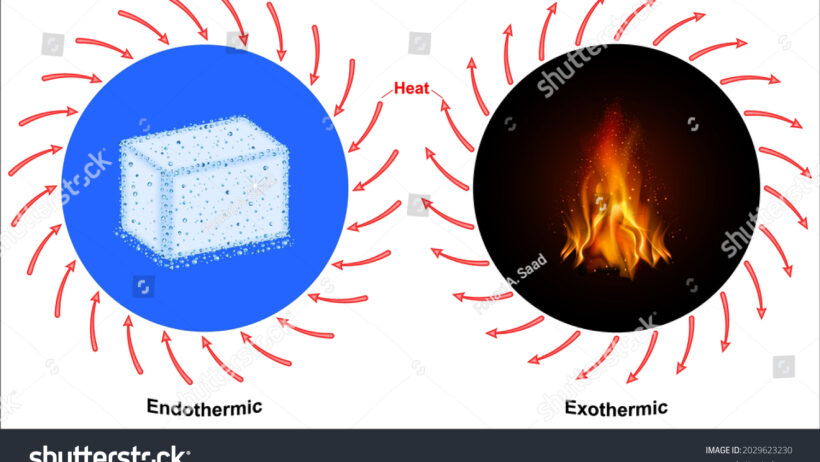
Before delving into the intricacies of energy conservation within these reactions, it is essential to clarify what endothermic and exothermic reactions entail. Endothermic reactions are akin to a parched desert craving for rain. In these reactions, energy—in the form of heat—is absorbed from the surroundings. Imagine, if you will, a sponge soaking up water; as it expands, it draws moisture from the air. By contrast, exothermic reactions are reminiscent of a hearth crackling with warmth. Here, energy is released, often in the form of heat, into the environment, much like a bonfire sharing its glow with a starry night. Each reaction type operates within the realm of thermodynamics, underscoring the conservation of energy—the total energy within a closed system remains constant, shifting between forms.
The Conservation Principle
The law of conservation of energy, or thermodynamic equilibrium, posits that energy can neither be created nor destroyed; it merely changes from one form to another. This universal principle is the bedrock upon which both endothermic and exothermic reactions rest. In essence, the energy absorbed by an endothermic reaction is subsequently equal to the energy released in an exothermic reaction, establishing a delicate balance. The concept is reminiscent of a pendulum, swinging fluidly between extremes, never losing momentum, just transforming it.
Endothermic Reactions: The Absorbers
Endothermic reactions absorb thermal energy, often leading to a drop in the surrounding temperature. A quintessential example is the process of photosynthesis, wherein plants harness sunlight to convert carbon dioxide and water into glucose and oxygen. Nature rejoices in this grand synthesis, seizing the sun’s radiant energy and converting it into chemical potential energy stored within glucose molecules. Thus, energy is conserved through the transformation of light into food, illustrating both innovation and conservation inherent in our ecosystems.
Another suitable analogy is a lone traveler on a journey through an expansive desert; the traveler represents the endothermic reaction, persistently seeking sustenance from the surrounding environment. As heat travels to the reaction, it echoes the collaboration between nature’s elements, retracing energy’s pathway as it crystalline forms of compounds emerge.
Exothermic Reactions: The Emitters
Conversely, exothermic reactions are exuberant and lively, akin to a celebratory feasting as they liberate energy into their surroundings. Combustion reactions serve as a prime example, where fuels such as hydrocarbon gas release significant heat when consumed. It is a vibrant transaction between reactants and products, exuding warmth and light. These reactions demonstrate the dichotomy of energy forms, where the stored potential energy of chemical bonds transforms into kinetic energy, a force that often powers engines and heats homes. The flaring flame is the crescendo of energy release—a moment of transformation that encapsulates movement and power.
Once again, an intriguing metaphor resurfaces: consider a concert where musicians (reactants) harmonize to produce a symphony (products). Each note released translates into a palpable energy that resonates with the audience (the environment). Energy radiates outward, capturing attention and enriching the environment, delineating the cyclical nature of energy conversions.
Energy Transfer and Entropy
When examining the flow of energy in both endothermic and exothermic reactions, one must grapple with the concept of entropy—an essential measure of disorder in a system. The second law of thermodynamics asserts that in every energy transaction, there is a tendency toward increased entropy, moving toward a state of chaos. In exothermic reactions, this tendency can be observed as thermal energy disperses into the environment, increasing the overall disorder of molecular arrangements. The heat released entices nearby particles to vibrate and move, ultimately enhancing the degree of randomness in the vicinity.
On the other hand, in endothermic reactions, the cooling of the surroundings results in a localized decrease in entropy. As energy is absorbed, the transformation fosters a unique order, creating new molecular structures. This moment of organized chaos creates a paradoxical beauty, illustrating that while energy flows and changes form, it still adheres to the laws governing the universe.
Concluding Thoughts: The Energy Ballet
The story of energy conservation in endothermic and exothermic reactions showcases an intricate ballet of exchanges. It is both an artistic and scientific metaphor of give-and-take that echoes through the universe, defining the essential interplay of energy and matter. Just as nature articulates this fundamental principle through countless chemical processes, so too do we, as stewards of the planet, witness the implications of energy transformations in our daily lives.
Ultimately, acknowledging the dance between endothermic and exothermic reactions enriches our understanding of the larger narrative of energy. This understanding bears implications for addressing the climate crisis, feeding our aspiration to conserve energy and adopt sustainable practices. It reminds us that every reaction is not merely a product of heat but a testament to balance, resilience, and the conservation of life’s most indispensable resource—energy.