Have you ever wondered why a puddle disappears on a warm day? What happens to the water? Is it simply gone forever? In the world of science, answers often reveal more complexity than we first expect. The transformation of water from a liquid to a gas, a process known as evaporation, illustrates the principles of mass and energy conservation in striking ways.
To understand how both mass and energy are conserved during evaporation, we must delve into the underlying science of phase changes. Matter exists in different states, primarily solid, liquid, and gas. These phases are defined by the arrangement and energy of molecules. In solids, molecules are closely packed in ordered structures. In liquids, the arrangement becomes more looser, allowing molecules to move past one another. As energy is absorbed into the system, molecules gain kinetic energy, which then facilitates phase changes. Evaporation stands as a quintessential example of this phenomenon.
At its core, evaporation occurs when individual molecules at the liquid’s surface gain enough energy to break free from their intermolecular bonds. This energy often comes from heat. As the surrounding temperature rises, some molecules become energized and transition into the gaseous phase. Importantly, during this transition, the total mass of the water remains unchanged despite the apparent disappearance. Mass conservation, a fundamental tenet of classical physics, asserts that in a closed system, mass cannot be created or destroyed; it can only change forms.
Let’s take a deeper dive into the interplay of mass conservation. In a contained environment, the water molecules that escape into the air—while contributing to the vapor phase—still exist in the environment. They disperse into the atmosphere but remain as water vapor. Hence, if you were to capture all the vapor that has evaporated and condense it back into liquid form, you would retrieve the initial mass of the water. This is a demonstration of the law of conservation of mass in action.
On the other hand, energy conservation follows a similar narrative. As water molecules absorb energy, they do not gain mass, but their energy state changes significantly. Heat energy is converted into molecular kinetic energy, causing the molecules to speed up and break their hydrogen bonds. This energy transformation is essential for the evaporation process—no energy means no transition. During evaporation, the energy that has been absorbed by the molecules facilitates their migration from a denser liquid state into a lighter gaseous state.
Yet, one could pose a crucial question: What drives this absorption of energy, and how does it relate to environmental factors? The answer lies in various atmospheric conditions, including temperature, humidity, and wind. For instance, higher temperatures increase the kinetic energy of water molecules, accelerating evaporation rates. In contrast, high humidity inhibits this process, as the air is already saturated with water vapor. Wind, meanwhile, can help disperse the vapor, effectively reducing humidity and promoting further evaporation. These interactions illustrate the delicate balance of forces affecting the water cycle and larger climate systems.
The conservation of energy also extends beyond the immediate phase change. In various ecosystems, the energy from the sun is vital for driving the evaporation process. Solar energy heats bodies of water, fueling evaporation and subsequently contributing to the formation of rainfall. This continuous cycle underscores the harmonious interdependence of energy and matter—an equilibrium that sustains life on Earth.
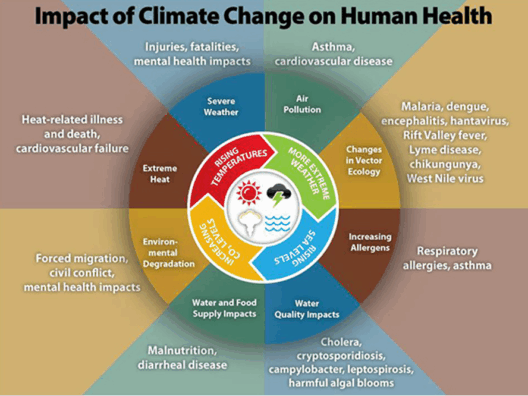
However, the implications of water evaporation extend beyond mere chemistry and physics; they hold profound significance for climate dynamics. The water vapor produced during evaporation acts as a greenhouse gas, influencing global temperatures and weather patterns. As climate change continues to disrupt traditional weather cycles, understanding evaporation is critical for addressing environmental challenges. Increasing temperatures augur higher evaporation rates, leading to exacerbated droughts and altered precipitation patterns in various regions.
What remains important in this interplay is how humans influence these natural processes. Urban development, deforestation, and industrial activities all alter local temperatures and humidity levels, thereby affecting evaporation rates. As mass and energy flow through these systems, the consequences ripple outward, impacting ecosystems, agriculture, and economies. Environmental stewardship has never been more pertinent than in this era of climate change. By harnessing a deeper understanding of fundamental processes like evaporation, we can craft innovative solutions that respect and restore ecological balance.
As we navigate the complexities of mass and energy conservation during evaporation, the theme of interconnectedness emerges. From the tiniest water molecule to the vast expanses of our planet’s atmosphere, each phase is integral to sustaining life. The playful question of what happens to a simple puddle under the sun reveals broader principles governing our world. One might challenge themselves to observe the next puddle they encounter: will it be dry in moments, or persist longer due to the conditions surrounding it? The answer lies not only in the science of evaporation but also in our stewardship of the environment.
The narrative of water evaporation transcends its scientific explanation; it poses questions about our environmental choices and responsibilities. By understanding and respecting the principles of mass and energy conservation within phase changes, we can contribute to a more sustainable future. Such knowledge empowers us to take action toward mitigating climate change and promoting environmental health, reminding us of the ongoing cycles that connect us all.