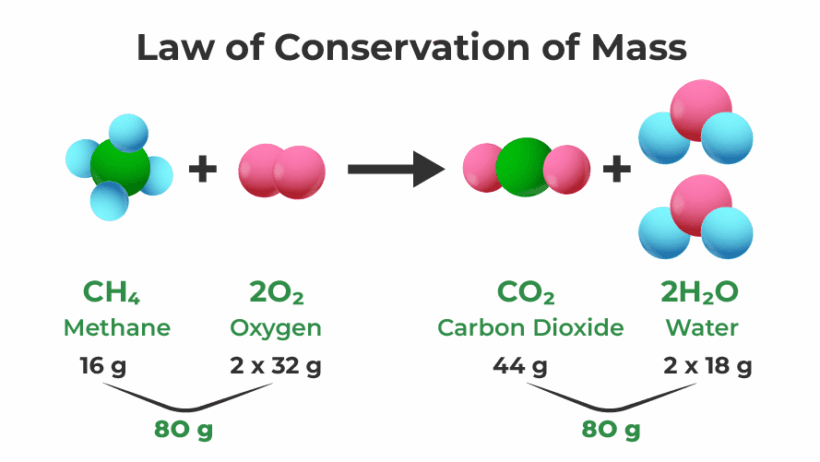In the vast tapestry of life, energy flows through every living organism, orchestrating a symphony of biochemical processes that sustain existence. Just as a comet streaks across the cosmos, leaving behind a trail of luminescence, energy in biology leaves an indelible mark on the ever-evolving landscape of life. The law of conservation of energy, a fundamental principle in physics, posits that energy cannot be created or destroyed; it can only transform from one form to another. This principle becomes a cornerstone in understanding the intricate dynamics of biological systems. The interplay of energy transference and transformation not only supports life but also fuels the ecological cycles that sustain our planet.
To fully grasp how the law of conservation of energy applies to biology, one must first appreciate the various forms of energy present in biological systems. From the sunlight that nourishes plants through photosynthesis to the chemical energy stored within the ATP molecules of our cells, energy manifests in ways essential for life’s continuity. Each organism, in its relentless quest for survival, engages in an elegant dance of conversion, transforming one type of energy into another to fuel its metabolic endeavors.
Consider, for instance, the process of photosynthesis, the biological equivalent of a masterful alchemist turning sunlight into sustenance. Green plants absorb photons from sunlight, converting this radiant energy into chemical potential energy stored within glucose molecules. This remarkable transformation is not a mere chemical experiment; it is a life-sustaining mechanism that fuels the cellular respiration of nearly all living organisms. The chemical bonds formed during photosynthesis represent a stored energy reservoir—vital for herbivores and, subsequently, for carnivores. Thus, sunlight, once captured and manifested as chemical energy, sustains entire ecosystems in a web of interdependence.
However, the energy does not remain static. It migrates and metamorphoses. The herbivore consumes the plant, converting the carbohydrates into energy through cellular respiration. Here, the law of conservation of energy is exemplified: as the plant’s chemical energy is broken down and transformed into kinetic energy, stored energy is released in the form of ATP, which powers cellular activities. The energy remains constant throughout these transformations, merely adopting different forms suitable for various life processes. This continuous flow of energy characterizes the resilience and interconnectedness of ecological systems, underscoring the necessity of balance in nature.
The transfer of energy is not limited to the confines of a single organism. Across ecosystems, energy flows from producers to consumers and ultimately to decomposers. The unique appeal of this energy transfer lies in its cyclical nature. Decomposers, such as fungi and bacteria, play a pivotal role by breaking down organic matter, recycling nutrients, and returning energy to the soil. This decomposition enriches the earth, fostering the growth of new plants and perpetuating the cycle of life. Here, one can visualize the process as akin to a grand relay race, in which energy is passed from one runner to another, each segment of the race contributing to a greater whole.
Yet, this elegant cycle is not immune to disruption. The introduction of anthropogenic factors has introduced a perturbation in the natural order, highlighting the fragility of this balance. The insatiable demand for resources, rampant deforestation, and pollution serve as stark reminders of our current ecological plight. These actions disrupt the flow of energy within ecosystems, hindering the processes of photosynthesis, respiration, and nutrient recycling. As energy becomes siphoned away from natural systems, a ripple effect ensues, compromising biodiversity and increasing vulnerability to climate change.
This dynamic relationship becomes even more apparent when we explore trophic levels within an ecosystem. Each step in the food chain acts as a transfer point, where energy diminishes due to inefficiencies in energy conversion. Approximately 90% of energy is lost as heat at each trophic level, leaving only a fraction available to the next consumer. This illumination of energy loss not only delineates the structure of food webs but also emphasizes the efficiency required for survival within this web of connections.
Moreover, in the realm of evolutionary biology, the law of conservation of energy shapes the pathways of adaptation and survival. Organisms have evolved efficient mechanisms to harness and utilize energy. From the intricate designs of photosynthetic machinery to the metabolic pathways that optimize energy production, life continually refines its strategies to thrive. It is a testament to the ingenuity of nature, crafting solutions rooted in the laws of physics, biology, and chemistry. The allure of these adaptations is not simply academic; they offer profound insights into how species can persist amidst the vicissitudes of environmental change.
In conclusion, the law of conservation of energy serves as a formidable concept in biology, intricately weaving through the threads of life. It encapsulates the essence of energy transformation and underscores the interconnectivity of ecosystems, emphasizing our collective role as stewards of this delicate balance. Understanding this concept not only enriches our appreciation of life’s complexities but also fortifies our resolve to combat the challenges posed by climate change. Embracing this knowledge allows us to engage more consciously with our environment, ensuring that the cycles of energy remain intact for generations to come. In our hands lies the potential to embody the principle of conservation, fostering a sustainable future where the energy of life can continue to flow unabated.