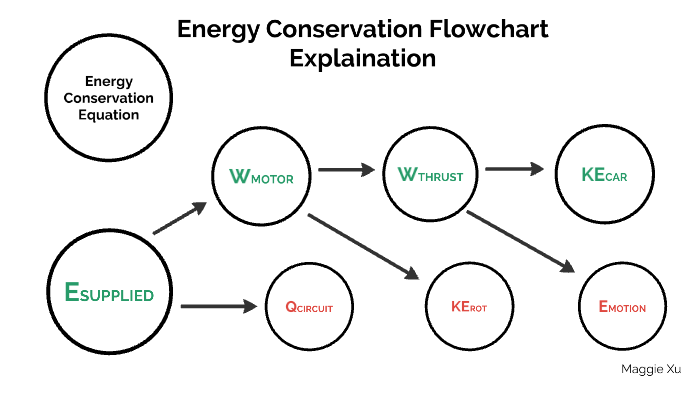
The Law of Conservation of Energy is a fundamental principle in physics which posits that energy cannot be created or destroyed; it can only be transformed from one form to another. This principle encompasses a wide array of phenomena and is critical for understanding various scientific disciplines, from physics to environmental science. Understanding this law requires navigating through several sub-concepts, real-world applications, and its implications in everyday life.
To delve deeper into the Law of Conservation of Energy, one must first understand its foundational elements. Energy exists in numerous forms, including kinetic energy, potential energy, thermal energy, chemical energy, and more. Kinetic energy, for instance, refers to the energy of motion. An object in motion, such as a speeding car, possesses kinetic energy that can be quantified. Conversely, potential energy is stored energy, such as a boulder resting on the edge of a cliff. When the boulder falls, its potential energy converts into kinetic energy as it accelerates towards the ground.
Energy transformations occur continuously in nature. Consider the process of photosynthesis in plants: solar energy from the sun is harnessed to convert carbon dioxide and water into glucose and oxygen. This transformation is a quintessential example of energy conversion that sustains life on Earth. The chemical energy stored in glucose can later be released via cellular respiration, providing necessary energy for organismal functions.
Another essential concept within this law is the principle of energy transfer. Energy transfer describes how energy moves from one system to another. For example, when a hot object comes into contact with a cold one, thermal energy transfers from the hotter object to the cooler one until thermal equilibrium is achieved. This transfer is pivotal in thermodynamics, highlighting the role of energy exchanges in creating the natural balance observed in ecosystems.
However, misunderstandings often arise regarding the meaning of energy conservation. The law does not imply that energy is stagnant or unchangeable. Instead, it emphasizes that while energy may change form or transfer from one entity to another, the total amount of energy remains constant in an isolated system. This principle is illustrated by the concept of energy efficiency and waste. When we use energy, such as combusting fossil fuels to generate electricity, only a portion is converted into usable energy; the remainder dissipates as heat, which is typically regarded as an energy loss. Optimizing energy use, therefore, becomes critical in mitigating waste and addressing climate change.
The implications of the Law of Conservation of Energy extend to various fields, particularly in engineering and environmental science. In engineering, energy conservation is paramount. Engineers design systems, be they engines, electrical circuits, or heating and cooling systems, informed by this law to maximize energy efficiency. Concepts such as renewable energy technologies—solar, wind, and hydropower—are predicated on leveraging natural energy sources to minimize reliance on finite resources, significantly affecting environmental sustainability.
In environmental science, the law plays a vital role in ecological studies by emphasizing the interconnectedness of natural processes. Ecosystems function through intricate webs of energy flow, where energy captured by producers (like plants) feeds into consumers (herbivores and carnivores) and decomposers. The energy’s journey through these food webs elucidates the necessity of every organism’s role, showcasing how disruptions (such as habitat destruction or pollution) can impact energy transfer and ecosystem stability.
Moreover, the concept has profound policy implications. Transitioning to a low-carbon economy is not merely an environmental cause; it involves rethinking how we produce and consume energy. This entails shifting from fossil fuels to renewable resources, enhancing energy efficiency in industries, and encouraging sustainable practices at the consumer level. Such shifts can reduce greenhouse gas emissions, contributing to a more sustainable and resilient planet.
Critically, we must recognize that while the Law of Conservation of Energy provides a theoretical framework, its practical application reveals the nuances and complexities of energy dynamics in real-world scenarios. Energy systems are characterized by inefficiencies and losses, and recognizing this fact is essential for devising effective strategies for energy management and climate action.
In conclusion, the Law of Conservation of Energy is integral to understanding the natural world and informing practical actions that can lead to a sustainable future. The ability to appreciate energy in its varied forms and the transformations it undergoes empowers individuals and communities to make informed decisions about energy use and sustainability. Thus, a thorough understanding of this law not only enhances scientific literacy but also promotes responsible stewardship of the planet amidst the pressing challenge of climate change.