Energy conservation is a principle that resonates throughout various scientific disciplines, echoing the tenets of thermodynamics. Within a closed system, one might philosophically liken energy to a treasure trove hidden beneath the surface of a tranquil lake, where the water represents isolation from external interactions. But is this treasure ever lost or merely transformed? This fundamental question invites us to explore the intricacies of energy conservation in closed systems.
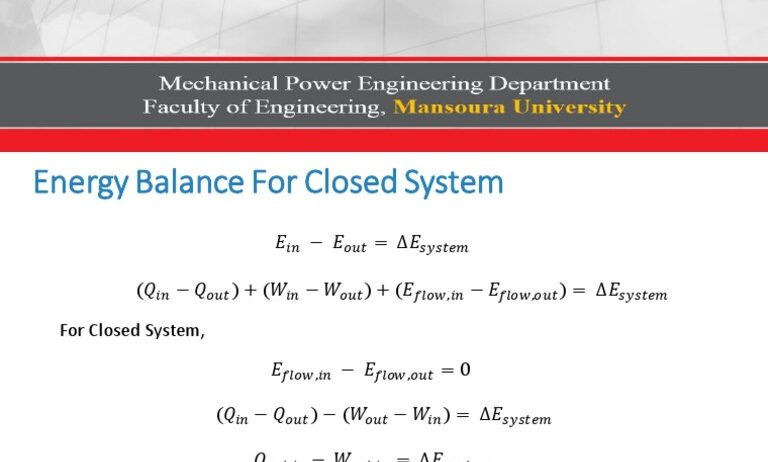
To unpack the concept of energy conservation, we must first delineate what constitutes a closed system. In thermodynamic terms, a closed system is defined as one that can exchange energy, yet not matter, with its surroundings. Imagine a sealed spaceship floating through space. Inside, all the oxygen, carbon dioxide, and other matter remain unchanged, yet the energy within the confines of that spacecraft is in a continuous state of flux. This situation underscores a critical point: while energy may change forms, its total quantity remains constant.
According to the First Law of Thermodynamics, also known as the law of energy conservation, the energy in a closed system can neither be created nor destroyed; it can only be transformed from one type to another. Consider a roller coaster ride as an analogy. At the highest point of the track, the energy is predominantly potential—positioned precariously at an apex. As the car hurtles downward, that potential energy metamorphoses into kinetic energy, exemplifying conservation in action. Similarly, in a closed system where no external forces influence it, energy simply shifts between forms while the total remains unchanged.
However, the nuanced reality of energy transfer can be further complicated by the Second Law of Thermodynamics, which introduces the concept of entropy. In any real process, the transformation of energy is never completely efficient, leading to wastage in the form of heat. Picture a luxurious tea kettle on the stove. As it heats, the sheer excitement of the boiling water tells a story of energy transfer. Yet, if you were to feel the kettle’s surface, it becomes evident that some energy has dissipated into the environment—not all of it contributes to the desired outcome of making tea. This inefficiency in the energy transfer subtly highlights that while energy remains conserved, its usability diminishes as systems evolve towards equilibrium.
One can hypothesize that, though energy retains its form and essence within a closed system, its efficacy may not persist eternally. The entropy generated indicates that while energy remains constant, the quality and the usefulness of that energy may degrade over time. Take the example of a car battery: charged with electrical energy, it effectively powers the vehicle until its capacity dwindles, ultimately reaching a point where it can no longer start the engine. This exemplifies how energy, while conserved, can lose the ability to perform work—a phenomenon indispensable in understanding energy conservation in closed systems.
Moreover, one fascinating aspect is energy transformations that occur within biological systems, highlighting the interplay between energy conservation and ecological dynamics. A forest ecosystem serves as a remarkable illustration. Sunlight is converted into chemical energy via photosynthesis, creating a complex tapestry of life. This energy circulates through the food chain, illustrating that while energy is conserved overall, it is perpetually in a state of evolution, intertwined with the health of the ecosystem. When examining closed ecological systems, it becomes evident that while energy is cycled through various forms, the total energy remains a constant, akin to a connected web of life.
Understanding energy conservation in closed systems also allows us to delve into technological advancements, paving the way for systematic innovations. Consider the development of heat exchangers in engineering. They are a practical application of the principles of energy conservation, capturing and repurposing waste heat in one part of a system for usage elsewhere—maximizing efficiency in line with conservation principles. Such innovations underscore the utility of recognizing energy transmutations rather than merely considering the static quantities of energy.
As humanity navigates the realities of a finite planet, the implications of energy conservation become increasingly critical. The increasing demand for energy, paired with finite resources, challenges us to rethink our interactions with energy systems. Embracing renewable energy sources, enhancing energy efficiency, and fostering sustainable practices reflect our understanding of energy conservation as not merely a scientific principle, but a vital tenet of environmental stewardship. This recognition of energy as a planet’s lifeblood emphasizes the need to honor its conservation, akin to preserving the essence of a natural spring amidst the encroaching urban tide.
In conclusion, energy is indeed conserved in a closed system, reaffirmed by the steadfast principles of thermodynamics. Nonetheless, the journey of energy is laden with transformations that shape its efficiency and applicability. As stewards of our shared earthly systems, we possess a responsibility to comprehend not only the constancy of energy but also the qualitative nuances that govern its practical use. By acknowledging both the unyielding nature of energy conservation and the perils of entropy, we can aspire towards a symbiotic relationship with our planet’s resources, crafting a future where energy is not only conserved but revered.