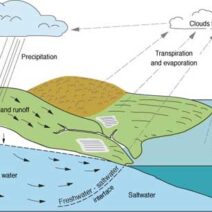
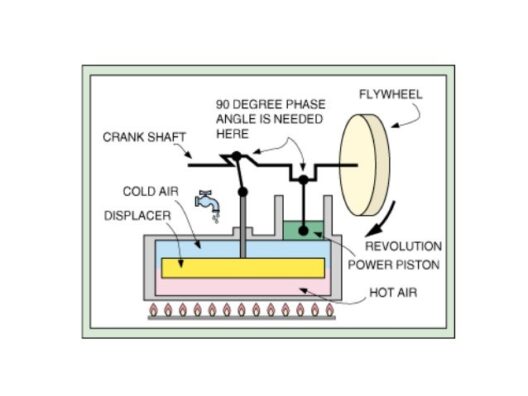
In the realm of physics, the conservation of energy and matter stands as one of the fundamental principles that underpin our understanding of the universe. The inquiry into whether energy and matter are always conserved together invites a plethora of discussions, explorations, and insights into both classical mechanics and modern theoretical constructs.
At the core of this discussion lies the law of conservation of energy, which asserts that the total energy in an isolated system remains constant. Energy can neither be created nor destroyed; it can only be transformed from one form to another. For instance, kinetic energy can be converted into potential energy, which is evident in activities as diverse as a pendulum swinging or water flowing over a dam.
Conversely, matter conservation, though similar, often elicits a slightly different interpretation. In classical physics, matter is perceived as a substance that occupies space and possesses mass. The law of conservation of mass declares that mass can neither be created nor destroyed; it can only change forms. This principle is crucial in chemical reactions, where reactants transform into products while maintaining the total mass of the system before and after the reaction.
The relationship between energy and matter conservation can lead to some intriguing conclusions, particularly when examining relativistic frameworks. According to Einstein’s theory of relativity, mass and energy are interchangeable, epitomized by the iconic equation E=mc². This relationship suggests that when mass is converted to energy, the overall quantity of mass and energy in a closed system remains invariant. Thus, while energy is transformed during nuclear reactions, such as in the core of a star or the fission process in a nuclear reactor, matter is likewise transformed into energy, maintaining the overarching conservation principle.
However, a distinction must be drawn between isolated systems and open systems. In isolated systems, both energy and matter are conserved in tandem. The classic example involves a closed container where both energy transformations and matter interactions occur. Yet, in open systems, where external forces and inputs come into play, determining the conservation of energy and matter can become more complex. The introduction of energy inputs—such as heat from a geothermal source—can alter the trajectory of energy conservation, although the mass remains invariant unless it escapes into the environment.
In the microscopic realm, quantum mechanics further complicates the conversation. The principles governing quantum particles challenge traditional perceptions of conservation laws. For example, in virtual particle theory, pairs of particles can spontaneously appear and annihilate each other, creating a fleeting balance between energy fluctuations and matter. While this phenomenon may appear to contravene conventional laws, it is crucial to comprehend that these interactions occur within the constraints of quantum fluctuations and adhere to the conservation laws on average over time.
Shifting perspectives, one can also examine conservation within ecological systems. Energy flows through ecosystems, wherein solar energy is captured by plants through photosynthesis, transformed into chemical energy, and subsequently transferred through various trophic levels. Here, the conservation of energy manifests not just as a principle of physics but as a foundational concept in understanding ecosystems’ dynamics and functions. While energy dissipates as heat through metabolic processes, the rest is cycled through the environment, highlighting nature’s pirouette of conservation.
Moreover, the synergy of energy and matter conservation can be illustrated through technological advancements and renewable energy sources. The imperative to harness energy sustainably has propelled innovations aimed at minimizing energy waste, such as solar panels and wind turbines. These technologies exemplify attempts to align with the conservation laws while promoting an ecosystem that thrives on renewable resources. They emphasize the wisdom of utilizing available matter and energy efficiently, reinforcing the interconnectedness of these two conservation principles in addressing environmental challenges.
In addressing potential contradictory scenarios, one must scrutinize the implications of dissipative processes. In practical applications like thermodynamics, while total energy is conserved, systems tend toward increased entropy or disorder over time. This phenomenon underscores that while the sum of energy and mass remains constant, their usable forms may diminish; thus, energy transformation processes can lead to inefficiencies and losses.
Furthermore, philosophical and ethical considerations must be integrated into discussions on conservation. The interconnectedness between energy and matter conservation relates closely to our societal responsibilities toward resource management and environmental stewardship. Understanding these conservation principles fosters a deeper appreciation for sustainability practices and encourages mindful consumption patterns in both personal and societal contexts.
In conclusion, while energy and matter are governed by distinct laws of conservation, their intricate connection cannot be understated. Energy may transform and manifest in myriad forms, while the mass remains consistent within isolated systems. Quantum terms demonstrate complexities that challenge our understanding yet reaffirm the overarching principles of conservation. As society continues to grapple with environmental challenges, recognizing these conservation dynamics can illuminate paths toward more sustainable practices, ultimately striving to align human activities harmoniously with the laws of the universe.