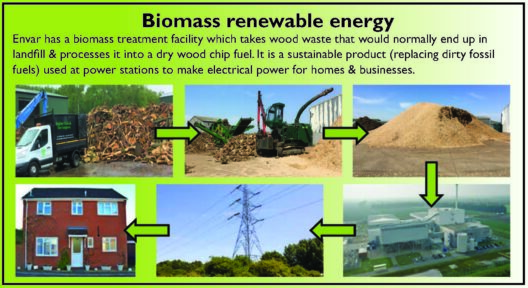
In the field of chemistry, exothermic reactions hold considerable significance due to their release of energy, often in the form of heat or light. It is essential to comprehend the underlying principles governing these reactions, particularly concerning the conservation of energy. This principle, known as the law of conservation of energy, posits that energy cannot be created or destroyed; it can only change forms. Within this context, we delve into the nuances of exothermic reactions, exploring whether energy is conserved in these specific scenarios.
Exothermic reactions are characterized by the release of energy. Common examples include combustion, respiration, and the thermite reaction. During such a reaction, the energy stored in the chemical bonds of the reactants is converted into thermal energy and released into the surroundings. The implication here is that while energy is emitted, the total energy within the closed system remains constant. Thus, energy is indeed conserved as it transitions from one form to another.
When analyzing exothermic reactions, it is crucial to distinguish between the total energy of the system and the energy that is released into the environment. For instance, in a combustion reaction, the reactants, such as hydrocarbons, undergo a chemical transformation in the presence of oxygen, leading to the formation of carbon dioxide and water. The chemical bonds in the reactants possess a certain amount of energy, which is higher than that of the products. This difference in energy levels explains why heat energy is emitted during the reaction.
To quantify this phenomenon, chemists often employ the concept of enthalpy (ΔH). Enthalpy change is a measure of heat content and serves as an invaluable tool for predicting whether a reaction will occur spontaneously. In exothermic reactions, the enthalpy change is negative, indicating that the reaction releases energy. Consequently, while the energy in the chemical bonds of reactants is transformed into thermal energy, the overall energy balance within the system remains intact.
Thermodynamics plays a pivotal role in understanding exothermic processes. The first law of thermodynamics, which is synonymous with the conservation of energy principle, states that the internal energy of a closed system changes when heat flows into or out of it, or when work is done on or by the system. In the context of an exothermic reaction, the system loses energy to the environment, confirming that energy is conserved overall.
Furthermore, it is pertinent to discuss the role of activation energy in exothermic reactions. Activation energy is the minimum energy required for a reaction to proceed. Upon surpassing this energy barrier, the reaction can occur, leading to the release of energy. While it may seem that energy is “lost” during this process, it is essential to recognize that this energy is merely being redistributed, reinforcing the conservation principle.
In practical application, exothermic reactions have profound implications in various domains. For instance, in the realm of energy production, the combustion of fossil fuels in power generation is a quintessential exothermic reaction. The chemical potential energy stored in hydrocarbons is converted into electrical energy, illustrating the transition of energy forms while adhering to conservation laws. Here, the challenge lies in managing the byproducts and environmental impact, as the by-products of combustion can contribute to air pollution and climate change.
Furthermore, the study of exothermic reactions extends into biological systems. Cellular respiration is a prominent example where glucose is oxidized to produce ATP (adenosine triphosphate), the energy currency of cells. The overall reaction releases energy, which is critical for sustaining life. In this biological exothermic process, the energy conversion is crucial for metabolic functions, thus underscoring the significance of energy conservation in living organisms.
Additionally, it is essential to consider the implications of exothermic reactions in industrial applications. Industries frequently harness exothermic reactions for purposes such as heating, welding, and material synthesis. Knowledge of energy conservation helps in optimizing these processes, enhancing efficiency, and reducing waste, contributing to more sustainable practices.
Beyond the immediate applications, exothermic reactions have sparked interest in sustainable technologies. For instance, researchers are exploring exothermic processes for energy storage and conversion, such as thermochemical energy storage. By capturing the heat released during reactions, these technologies aim to provide renewable energy solutions, demonstrating the potential for energy conservation to mitigate environmental impacts.
Ultimately, while exothermic reactions release energy into the environment, the conservation of energy principle remains steadfast. The energy transitions from stored chemical potential to thermal or kinetic energy while adhering to the laws of thermodynamics. Understanding this relationship is pivotal for various scientific, industrial, and ecological endeavors. With the knowledge of energy conservation in exothermic reactions, we can approach energy utilization with a greater sense of responsibility and innovation, ultimately fostering a more sustainable future.