In the natural world, energy plays a pivotal role in the wondrous tapestry of life and matter. A fundamental question arises in the context of chemical reactions: Is energy conserved in all chemical reactions in nature? This inquiry invites us to consider not just the mechanics of chemistry but also the philosophical implications of energy conservation and its role in ecological systems.
To tackle this question, we must first delve into the principles of thermodynamics, particularly the first law, which states that energy cannot be created or destroyed; it can only change forms. Whether chemical bonds are formed or broken, the total energy before and after a reaction remains constant. This principle underpins a myriad of chemical transformations, from the combustion of fuel to the intricate biochemical pathways in living organisms.
Consider the combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O. In this exothermic reaction, chemical potential energy stored in the bonds of the methane and oxygen molecules is released as thermal energy when new bonds are formed in carbon dioxide and water. The energy conservation principle is palpable—what starts as one form of energy transitions seamlessly into another. But does this principle hold true across all chemical reactions, from the simple to the complex?
While the first law of thermodynamics provides a robust framework, the concept of energy conservation evolves when we scrutinize specific types of reactions. Endothermic reactions, where external energy is absorbed, challenge our perceptions. Take, for instance, the process of photosynthesis: 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂. Here, plants capture solar energy to synthesize glucose, a paradoxical moment where energy appears to be ‘created’ from light. However, despite this seemingly miraculous transformation, the overall energy within the system does not violate conservation laws. It simply reveals the complex interplay of energy states, emphasizing that energy transformations can be multifaceted.
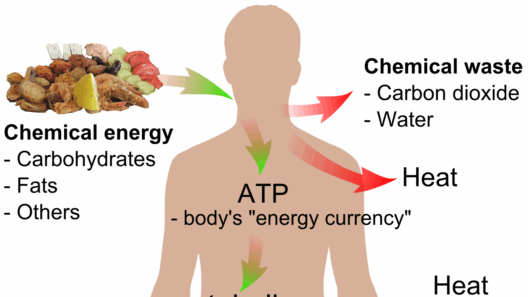
When considering biological systems, the conservation of energy takes on an even more intricate dimension. Metabolic pathways exemplify how organisms harness and utilize energy from their environment. Through a series of biochemical reactions, organisms convert nutrients into usable forms of energy; yet, energy transfer is replete with inefficiencies and losses, primarily as heat. As such, while energy itself is conserved across these reactions, the efficiency of conversion can vary vastly. Is this variability what complicates our understanding of energy conservation?
Moreover, the emergent concept of exergonic and endergonic reactions provides additional context. While exergonic reactions release energy and are spontaneous, endergonic reactions necessitate energy input to proceed. This duality exemplifies that, although energy is conserved, the manner in which it is utilized can yield reactions that seem to defy the conservation principles at first glance. This juxtaposition beckons a deeper exploration into the energetic landscape of chemical reactions.
Yet, not all chemical interactions abide by these simplified paradigms. In the realm of nuclear chemistry, the conversion of mass to energy can be observed, epitomized by Einstein’s famous equation, E=mc². Nuclear fission and fusion processes yield energy far beyond what is typically witnessed in conventional chemical reactions. Here, the conservation laws gravitate towards modifications, as a fraction of mass is indeed transmuted into energy, raising the question: Can we consider these exceptions as mere outliers, or do they signify a broader principle at play? This invites further philosophical discussion regarding the rigidity of conventional energy conservation laws.
The implications of energy conservation extend beyond academic inquiry into realms of policy and environmental stewardship. Understanding the mechanisms of energy transfer and transformation is vital in the quest for sustainability. As we grapple with challenges such as climate change and resource depletion, harnessing energy efficiently becomes imperative. By acknowledging the nuances of energy conservation in chemical reactions, we empower ourselves to innovate and create systems that align with sustainable practices.
Moreover, the significance of energy conservation touches upon fundamental ethical considerations. In a world where resources are finite, the question poses itself: how do we balance the conservation of energy within chemical processes against our needs and desires? The delicate equilibrium between energy consumption and conservation becomes a central theme in the discourse surrounding environmental responsibility.
In conclusion, while energy is fundamentally conserved in chemical reactions, the complexity and variances in different types of reactions necessitate a nuanced understanding of how energy transitions between states. From combustion reactions to metabolic pathways and even nuclear processes, energy remains a constant companion, guiding the flow of chemical transformations. The essential quandary, “Is energy conserved in all chemical reactions in nature?” serves not only as a scientific inquiry but also as a clarion call for evaluating our interactions with energy and the planet. A deeper understanding of these interactions holds the key to developing sustainable practices that honor the conservation of energy while promoting the well-being of our environment.