The subject of energy conservation in the context of radioactive decay raises intriguing questions that integrate the realms of physics, environmental science, and the fundamental laws governing the universe. When examining whether energy is conserved or transformed during radioactive decay, it’s essential to unravel not just the scientific principles involved but also the implications for energy conservation and environmental awareness.
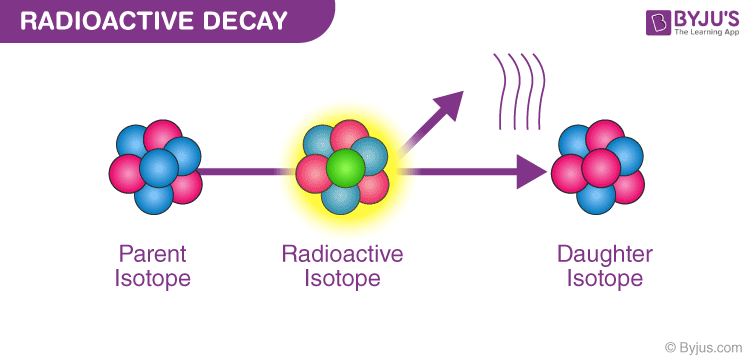
Radioactive decay is a spontaneous process by which unstable atomic nuclei lose energy by emitting radiation. This decay can manifest in various forms—beta decay, alpha decay, and gamma decay, which play critical roles in our understanding of nuclear physics and energy transformations.
At its core, the principle of energy conservation states that energy cannot be created or destroyed; it can only change forms. In the case of radioactive decay, it might be apparent that energy appears to be lost as radiation is emitted. However, what occurs is a transformation of energy. The energy that is released during decay stems from the conversion of mass to energy, as articulated by Einstein’s famous equation, E=mc². This equation expresses that mass can be converted to energy, adhering to the conservation principle yet highlighting an intricate transformation.
Examining the processes involved in radioactive decay, we find that in alpha decay, an unstable nucleus ejects an alpha particle (composed of two protons and two neutrons). This ejection results in a new nucleus with a lower mass that retains some energy. The energy released in this process can be harnessed, as alpha radiation carries significant energy that can be converted to usable power in certain contexts.
In contrast, beta decay involves the transformation of a neutron into a proton, emitting a beta particle and an antineutrino. This decay process not only alters the atomic mass and atomic number of the element but also releases a considerable amount of energy, once again adhering to the principles of mass-energy equivalence. The energy emitted is absorbed as kinetic energy by the particles involved in the decay, illustrating the transformation rather than a loss.
Gamma decay is another fascinating phenomenon where an unstable nucleus releases excess energy in the form of electromagnetic radiation without a change in atomic number or mass. This high-energy gamma radiation can penetrate through various materials, indicating a substantial release of energy. Again, the energy in gamma decay serves as an illustration of the transformation of a system striving for stability.
These nuclear reactions form the basis of our understanding of not just atomic behavior but also the broader implications of energy generation. For instance, the principles governing radioactive decay are applied extensively in nuclear energy production. Nuclear reactors utilize controlled radioactive decay to produce power, emphasizing the practical applications of these processes and offering insight into energy transformation practices.
Despite the potential for harnessing energy through nuclear processes, it is imperative to address the environmental concerns surrounding radioactive decay and nuclear power. The long-lived radioactive isotopes in nuclear waste pose significant challenges for energy conservation and environmental restoration. The decay of these isotopes emits radiation, which can have harmful effects on living organisms and ecosystems. Therefore, while energy is technically conserved in radioactive decay, the transformation into radiation leads to environmental repercussions that must be meticulously managed.
When considering alternative energy sources, it’s crucial to recognize the balance between harnessing nuclear energy’s potential and mitigating its harmful impacts. As nations grapple with the challenge of reducing carbon emissions and combating climate change, understanding the nuanced role of radioactive decay and energy transformation will contribute to informed policy decisions regarding energy conservation and responsible resource management.
Additionally, the conversation around radioactive decay extends to medical applications as well. Radioactive isotopes are employed in treatments and diagnostic procedures, such as cancer therapies and imaging. In these applications, energy is not only conserved; it is applied in innovative ways to enhance human health, reflecting a more positive transformation of energy in real-world contexts.
Moreover, developments in environmental policies and green energy initiatives underscore a growing recognition of diverse energy forms and their implications on conservation efforts. By integrating knowledge of radioactive decay with contemporary environmental challenges, we can foster a holistic understanding of energy systems, their transformations, and their ecological footprints.
In conclusion, when analyzing the conservation and transformation of energy in radioactive decay, it becomes evident that energy does not vanish but rather shifts forms, aligning with the core principles of physics. Through various decay processes, energy is transformed into radiation and other forms, each bearing distinct implications for both scientific inquiry and environmental stewardship. The dual nature of radioactive decay—its ability to serve as an energy source while posing environmental risks—merits careful consideration as society navigates the complexities of energy conservation and development in a rapidly changing world.