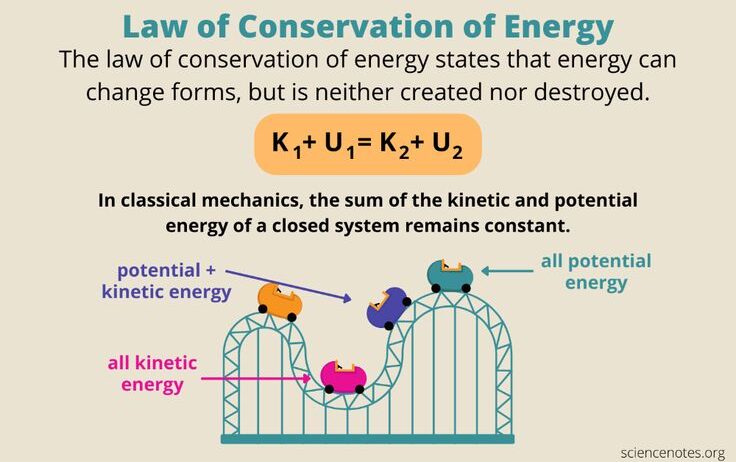
The First Law of Thermodynamics, often encapsulated in the adage “energy cannot be created or destroyed, only transformed,” is a fundamental principle governing the interactions of energy and matter in the universe. At first glance, this might appear straightforward, suggesting a mere conservation of energy. However, a deeper examination reveals complexities that underscore not only the law itself but also its implications for various scientific fields and everyday phenomena.
At the core of the First Law is the concept of energy transfer. Energy exists in myriad forms, including kinetic, potential, thermal, and chemical energy, among others. These various forms can be converted from one to another. For instance, when a hydroelectric dam converts gravitational potential energy into electrical energy, it exemplifies this transformation. Such mechanisms are not trivial; they point to the intricate stability of energy states within a system while revealing the transitional dynamics that often go overlooked.
The law is rooted in empirical observations made through centuries of scientific inquiry. Early scientists, such as calorimetrist Joseph Black, laid foundational work by demonstrating that heat is a form of energy. Ultimately, these observations coalesced into a broader understanding captured in the First Law, which is fundamentally an articulation of the conservation principle. Its importance goes beyond thermodynamics and is pivotal in fields such as chemistry, biology, and even ecology.
In chemistry, for instance, the conservation of energy implies that during chemical reactions, the total energy of the reactants must equal the total energy of the products, albeit in different forms. This relationship is crucial for predicting the outcomes of reactions, informing everything from industrial manufacturing processes to metabolic pathways in living organisms. By understanding how energy shifts during chemical changes, scientists can harness energy more efficiently, usually a critical step towards sustainability.
Biological systems, too, rely heavily on the principles enshrined in the First Law. An organism’s metabolism illustrates this beautifully: energy stored in food is transformed into kinetic energy for movement or potential energy for growth. The intricacies involved in these transformations highlight the delicate balance of energy flows essential to life. An organism that efficiently captures and transforms energy is more likely to thrive, underscoring the significance of energy conservation in the evolutionary narrative.
Ecologically, the First Law has profound implications. Ecosystems are defined by their energy exchanges, from sunlight captured by plants through photosynthesis to the energy transferred through various trophic levels. The dynamic interplay of energy within an ecosystem dictates its health and functionality. Disruptions to this balance—e.g., habitat loss, pollution—often lead to diminished energy flows and reduced biodiversity. Understanding how energy conservation operates within these systems holds the key to both preserving biodiversity and developing sustainable practices. Essentially, the First Law acts as the frame of reference through which ecological interactions can be understood and monitored.
What is particularly fascinating about the First Law of Thermodynamics is its universality. This principle not only governs physical and chemical processes here on Earth, but it applies on cosmic scales as well. For instance, the lifecycle of stars—where mass is converted into energy through nuclear fusion—can be described using thermodynamic principles. Explorations of stellar phenomena convey an awe-inspiring depth of energy exchange, regularly transitioning between matter and energy, as might have occurred during the Big Bang. These cosmic narratives add layers to our fascination with energy conservation.
Yet, the implications of the First Law prompt contemplation beyond the realm of science. They provoke philosophical inquiries about our existence. The inevitability of energy transformations can incite reflections on sustainability in our everyday lives. As humanity grapples with climate change and the depletion of natural resources, understanding energy conservation becomes paramount. It conveys a message about responsibility—encouraging us to rethink and optimize how we consume and preserve energy resources.
The principle embodies more than a scientific law; it serves as a necessary lens through which we can anticipate and mitigate the effects of human activity on natural ecosystems. It’s this intersections of science and stewardship that captivates individuals, highlighting the moral imperative to embrace more sustainable practices in energy consumption. For example, adopting renewable energy sources, such as solar or wind power, epitomizes a practical application of the First Law—maximizing energy transformations while minimizing waste.
Moreover, the law provides critical insights into the design of energy-efficient systems. Engineers and scientists are increasingly tasked with creating devices that optimize energy conversions, thereby reducing waste and increasing efficiency. Hybrid vehicles that capture braking energy and convert it into usable power are tangible outcomes of such efforts. These innovations underscore a proactive approach to energy conservation, illustrating how the First Law transcends theoretical boundaries and informs practical advancements.
In conclusion, while the First Law of Thermodynamics is fundamentally about the conservation of energy, its broader ramifications reverberate through various realms of existence—including chemistry, biology, ecology, and even philosophy. Each transformation of energy carries implications, shaping the natural world and highlighting the necessity for a conscientious approach to energy use. In a landscape marked by climate challenges, understanding and embracing the principles of energy conservation is not merely a scientific mandate—it is a call to action for humanity. The awe-inspiring unfurling of energy’s endless cycles invites curiosity and evokes responsibility toward both our immediate environments and the vast universe beyond.