Have you ever wondered what keeps a roller coaster soaring through loops without an engine propelling it forward? Can energy really be “saved” like money? These questions lead us down the fascinating path of energy conservation in physics, a fundamental principle underpinning countless phenomena in our universe. In this beginner’s guide, we will explore the intricate meanings of energy conservation, the fundamental laws of thermodynamics, and the practical implications of this vital concept.
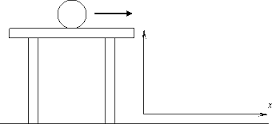
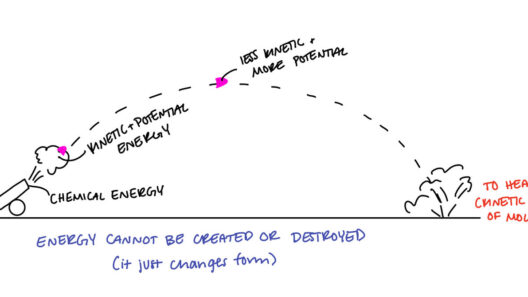
The principle of conservation of energy asserts that energy cannot be created or destroyed, only transformed from one form to another. This axiom, rooted in classical mechanics, suggests that in a closed system, the total amount of energy remains constant. Imagine a swinging pendulum. At the apex of its swing, all the energy is gravitational potential energy. As it descends, that potential energy converts into kinetic energy, reaching its maximum when it swings through the lowest point. When revisiting the top on the other side, energy converts back to potential energy once again. This ongoing oscillation epitomizes the conservation of energy in action.
At its core, this principle is interconnected with the first law of thermodynamics. This law postulates a relationship among internal energy, heat transfer, and work done in a system. It emphasizes that while energy transforms from one form to another—thermal, kinetic, potential—its total aggregate remains unchanged. The myriad forms of energy—mechanical, chemical, electrical, thermal—can engage in a complex dance, yet they adhere to this enduring constraint.
Consider the simple act of boiling water. When you place a vessel over a flame, chemical energy from the fuel converts into thermal energy, heating the water. The water molecules become agitated, transforming thermal energy into kinetic energy as they vaporize. Remarkably, the energy inputs and outputs are calibrated, ensuring a perpetual balance. This dynamic showcases how energy transitions while maintaining a conserved state.
But what are the implications of this principle? In real-world applications, the conservation of energy shapes the very foundation of engineering, mechanics, and even environmental science. Would it surprise you to know that energy inefficiency is not just an inconvenience but a critical challenge facing modern society? Exploring renewable energy sources and sustainable practices inherently ties back to the principle of energy conservation. We harness wind, solar, and hydro energy by effectively converting these forms into usable electrical energy without exceeding the natural energy availability of our planet.
This leads us to another fascinating aspect: the law of energy degradation or entropy. Associated with the second law of thermodynamics, it states that while energy within an isolated system remains constant, the quality and usability of that energy deteriorate over time. For instance, when energy is transformed, not all of it remains in a form that can perform work. In a car engine, chemical energy from fuel transforms to kinetic energy to propel the vehicle; however, some energy dissipates as heat. As entropy increases, more energy becomes “lost” to the environment, indicating that conservation is not merely about retaining quantities but optimizing quality.
As we delve deeper, let’s consider playful thought experiments. Imagine if energy conservation didn’t exist. If energy could be created at will, would we live in a utopia filled with perpetual motion machines? The ramifications on our economy, society, and environment would be profound. Producing energy without limitations could lead to rampant exploitation of resources, indulge in extravagant consumption, and ultimately foster an unsustainable existence.
To encapsulate the relevance of energy conservation, the challenges emerging from climate change and resource depletion place immense pressure on our understanding of energy management. Acknowledging that our resources are finite compels us to regard energy not just as a commodity but as a shared responsibility. This perspective informs global initiatives towards cleaner energy transitions and innovations in efficiency, further reinforcing the significance of conserving energy.
However, one might ask, how can individuals apply the principle of energy conservation in their daily lives? Simple actions can yield notable outcomes: using energy-efficient appliances, engaging in responsible consumption, and exploring alternative transportation methods like cycling or public transit. These practices contribute to a collective effort towards a sustainable future and exemplify the practical application of energy conservation.
Moreover, educational institutions play a critical role by instilling awareness from a young age. The next generation must grasp not just the theoretical aspects of energy conservation, but its tangible relevance in promoting environmental stewardship. Engaging in experiments with renewable energy sources, such as building solar ovens or wind turbines, can illuminate the fascinating interplay of energy transformations, while also serving to foster a deeper respect for finite resources.
In conclusion, the conservation of energy is an elemental tenet of physics that permeates various aspects of life and nature. It impressively illustrates the interconnectedness of energy forms and the consequences of their transformation. As we face the imminent challenges of climate change and the ceaseless demand for resources, our understanding and application of energy conservation become imperative. Whether you are a student, an engineer, or simply an environmentally-conscious individual, grasping the principles of energy conservation can inspire responsible choices leading towards a more sustainable universe.
So, the next time you turn off a light or choose a bicycle over a car, remember the significance behind those actions. They echo the fundamental laws of nature—energy cannot be destroyed; it can only be repurposed or lost. The choice is yours to make it a positive transformation.