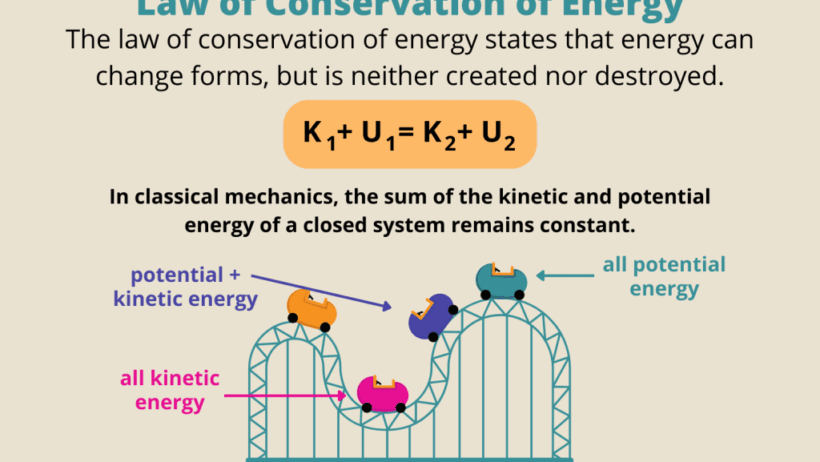
The Law of Conservation of Energy is a fundamental principle in physics that states energy cannot be created or destroyed; rather, it can only be transformed from one form to another. This law underpins a myriad of scientific phenomena and is essential in understanding various systems within our universe. To grasp the implications of this law, it is crucial to explore different types of energy, examples of energy transformations, and the broader significance of energy conservation in modern society.
Energy comes in multiple forms, each playing a vital role in the functionality of our environment. The principal types of energy include kinetic, potential, thermal, chemical, electrical, and nuclear energy. Kinetic energy is the energy of motion, applicable to all objects in movement, from a bus driving down the street to electrons flowing in a conductor. In contrast, potential energy is stored energy, found in objects that are at rest but have the potential to move, such as a roller coaster at the top of a hill or a compressed spring.
Thermal energy, often termed heat, arises from the movement of particles within a substance. The hotter an object, the greater its thermal energy. Chemical energy resides in the bonds that hold atoms together within molecules, playing a crucial role in chemical reactions. For instance, the energy released when gasoline combusts in a car engine drives it forward. Electrical energy, another vital form, stems from the movement of charged particles, typically electrons, and powers our homes and electronic devices. Lastly, nuclear energy, harnessed in power plants and from radioactive materials, originates from the forces that bind protons and neutrons within atomic nuclei.
The transformation of energy from one form to another exemplifies the Law of Conservation of Energy effectively. A classic example occurs in a pendulum. When the pendulum is at its highest point, it possesses maximum potential energy. As it swings downward, this potential energy converts into kinetic energy, reaching its maximum as it swings through the lowest point. As it continues its path upward, kinetic energy transitions back into potential energy, illustrating energy transformation while adhering to conservation principles.
Other common scenarios that elucidate the law include the operation of a hydroelectric power plant. Water stored in a reservoir represents potential energy. When released, it flows downward, converting potential energy into kinetic energy capable of turning turbines. The turbines convert this kinetic energy into electrical energy, which we utilize daily. This cycle demonstrates how energy perpetually transitions through various forms while remaining conserved. None of the energy is lost; instead, it changes form according to our operational needs.
The implications of the Law of Conservation of Energy extend far beyond basic physics. Energy conservation is pivotal in addressing contemporary challenges, particularly in relation to climate change and resource sustainability. As societies strive to reduce their carbon footprint, energy efficiency emerges as a key strategy. By maximizing the utility of energy—whether through improved technologies, enhanced insulation in buildings, or transitions to renewable energy sources—we can mitigate environmental impacts while adhering to the fundamental principles of energy conservation.
In addition to addressing climate issues, understanding energy conservation principles aids in revolutionizing technologies across various sectors. The development of electric vehicles (EVs) illustrates this concept vividly. By capitalizing on electric energy from batteries, EVs convert stored chemical energy into kinetic energy while minimizing heat waste compared to traditional combustion engines. Optimizing energy transfer enhances the overall efficiency and promotes a more sustainable future.
Moreover, the concept of energy conservation invites a holistic examination of systems within nature, economics, and social structures. Ecosystems are prime examples where energy flows from one form to another. During photosynthesis, plants convert solar energy into chemical energy, producing glucose—a vital component for life on Earth. This process underscores the interconnectedness of energy transformations in sustaining ecological balance, illustrating that the principles governing energy are universal and far-reaching.
In a broader socio-economic context, energy conservation encourages innovative practices such as recycling and resource reuse. The processing of materials in manufacturing often demands vast amounts of energy, leading to significant environmental degradation if not managed properly. By recycling materials, we conserve the energy that would otherwise be expended in extracting and processing new raw materials, echoing the law that emphasizes energy transformation rather than loss.
Educational initiatives emphasizing the Law of Conservation of Energy can significantly influence future generations’ understanding of sustainability. School curriculums that incorporate practical experiments demonstrating energy transformations can enhance early awareness of energy principals. Through hands-on learning experiences, students grasp the concept more profoundly, fostering a culture of conservation and respect for natural resources as they grow into informed adults.
In conclusion, the Law of Conservation of Energy serves as a cornerstone in the understanding of physical science and our world. It provides clarity on how energy perpetuates, transforming between forms without loss. From practical applications in technology and natural ecosystems to broader implications in sustainability and education, this principle influences countless facets of modern life. By embracing and elucidating energy conservation, society can pursue a path toward a more sustainable, resource-efficient future, ultimately harmonizing technological advancement with the preservation of our planet.