The conservation of energy is a pivotal concept in physics that underpins various phenomena observed in the natural world. It asserts that energy cannot be created or destroyed; rather, it can only be transformed from one form to another. This principle elucidates the behaviors of mechanical systems, thermal processes, and even biological systems, providing a fundamental framework for understanding the physical universe.
To grasp conservation of energy comprehensively, it is essential to delve into several key components.
1. Definition of Energy
At its core, energy is defined as the capacity to perform work. It manifests in different forms, including kinetic energy (energy of motion), potential energy (energy stored due to position), thermal energy (energy related to temperature), chemical energy (energy stored in molecular bonds), and nuclear energy (energy released during nuclear reactions). Understanding these disparate forms is critical as they interchange throughout various processes.
2. Types of Energy
The diversity of energy types is instrumental in applying the conservation principle concretely. Each type has unique characteristics and roles:
- Kinetic Energy: This is the energy associated with moving objects. The kinetic energy of an object can be calculated using the formula KE = 1/2 mv2, where m is mass and v is velocity.
- Potential Energy: This energy type is stored energy, related to the height of an object above ground level. It follows the formula PE = mgh, where m is mass, g is the acceleration due to gravity, and h is height.
- Thermal Energy: Arising from the motion of particles, thermal energy increases with temperature. It plays a crucial role in heat transfer processes and thermodynamics.
- Chemical Energy: Found in chemical bonds, this energy is released during chemical reactions, such as combustion or metabolism.
- Nuclear Energy: This energy results from nuclear reactions, where energy is released by fission or fusion processes.
3. The Principle of Conservation
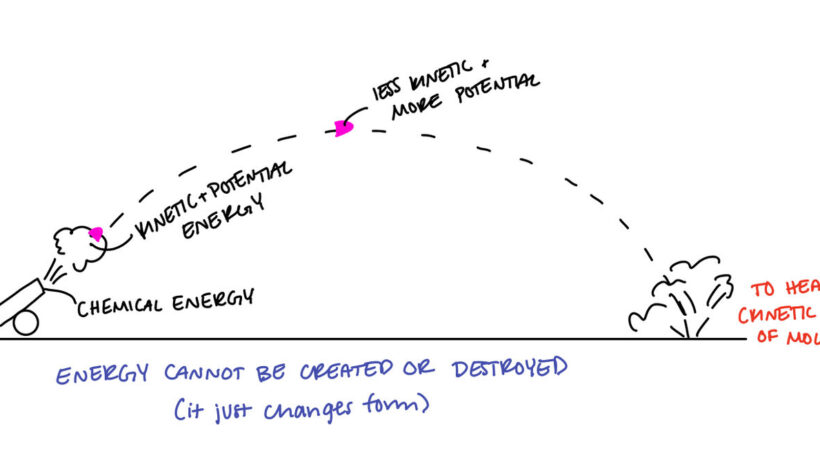
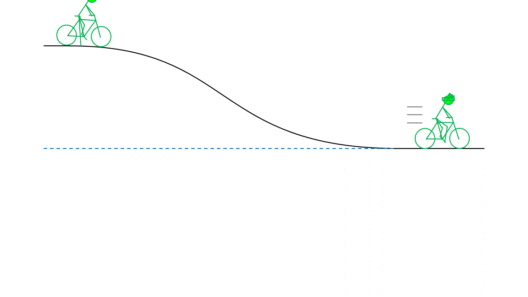
The principle of conservation of energy asserts that the total energy in a closed system remains constant over time. In practical terms, this means that if one form of energy decreases, another form must increase by an equivalent amount. This postulate is foundational in mechanics, where the total mechanical energy (kinetic plus potential) of a system remains constant if only conservative forces act upon it, exemplified by the classic example of a pendulum swinging back and forth.
4. Applications of Energy Conservation
Energy conservation principles are not confined to theoretical physics. They have practical applications across various fields:
- Engineering: Understanding energy conservation is paramount in designing efficient systems, from automobiles to buildings. Engineers apply these principles to minimize energy loss and enhance performance.
- Environmental Science: Energy conservation plays a monumental role in advocating for sustainable practices. It aids in the analysis of energy consumption patterns and promotes renewable resources that less impact the environment.
- Everyday Technologies: Advances in technology, such as hybrid vehicles and energy-efficient appliances, are grounded in the concept of conserving energy. This leads to substantial economic and environmental benefits.
5. Conservation of Energy in Isolated Systems
An isolated system, where no energy enters or exits, adheres strictly to the conservation principle. For instance, when a roller coaster car is at its peak, it possesses maximal potential energy. As it descends, this potential energy converts to kinetic energy, reaching its peak motion at the lowest point. Throughout this experience, the total energy remains unaltered, showcasing the beautiful symmetry of energy transformation.
6. Real-World Implications of Energy Conservation
Across the globe, energy conservation emerges as a pressing concern, particularly in the context of global warming and resource depletion. Strategic energy conservation results in diminished fossil fuel consumption, reduced greenhouse gas emissions, and a sustainable approach to resource management. Energy audits, improving insulation in buildings, and transitioning to renewable energy sources are tangible implementations of conservation principles aimed at fostering a sustainable future.
7. The Laws of Thermodynamics
The conservation of energy ties intimately with the laws of thermodynamics. The first law states that energy in an isolated system is constant, affirming that energy may transform but does not vanish. The second law introduces the concept of entropy, elucidating that energy transformations are not 100% efficient. This highlights the inevitable loss within energy systems—information essential for any scientific analysis of energy efficiency.
8. Conclusion
In summation, the conservation of energy represents a cornerstone of physics, bridging theoretical understanding with practical applications. By studying the dynamic interconversions of energy forms, individuals can enhance their comprehension of the natural world and improve energy utilization practices. As society continues to grapple with energy issues, recognizing and applying the tenets of energy conservation becomes increasingly important. Through informed choices and innovative technologies, it is possible to adhere to the principles of conservation and foster a more sustainable future.