The phenomenon of climate change, an escalating crisis of our time, embodies a complex interplay of chemical reactions and atmospheric dynamics that warrant meticulous examination. Central to this discourse is the greenhouse effect — a natural process that is being exacerbated by anthropogenic activities. This essay will delve into the chemistry behind greenhouse gases, exploring how they function to trap heat within Earth’s atmosphere, subsequently leading to global warming.
To comprehend the intricacies of climate change, we first must define what greenhouse gases (GHGs) are. These gases, including carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and water vapor (H2O), naturally exist in our atmosphere and are key players in the greenhouse effect. They possess unique molecular structures that allow them to absorb and re-emit infrared radiation, a critical mechanism that delicately balances our planet’s temperature.
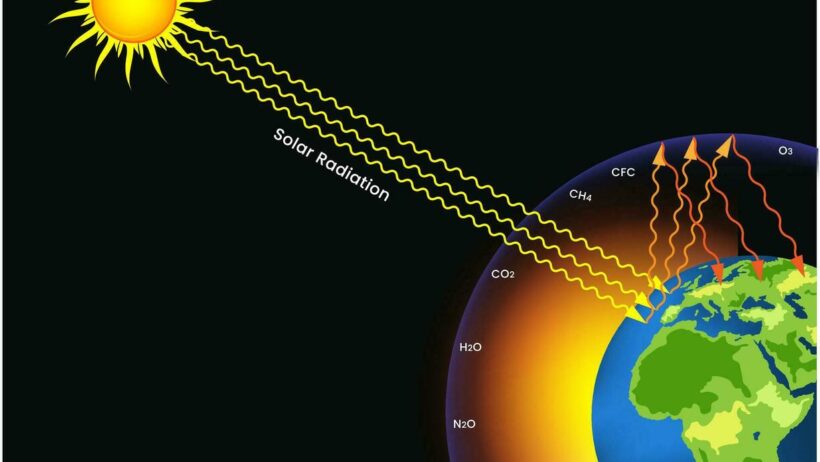
The greenhouse effect begins with the sun’s rays reaching the Earth’s surface. The Earth absorbs a portion of this solar energy and, as a result, warms up. This absorbed energy is then re-radiated in longer wavelengths as infrared radiation. Under normal circumstances, a significant portion of this heat would escape back into space. However, the presence of greenhouse gases complicates this process. These gases capture and re-radiate some of the infrared radiation, effectively trapping heat within the atmosphere. This retention of heat is vital for maintaining life as we know it, as it stabilizes temperatures across the globe.
Before delving deeper, it is essential to address an observation that may seem mundane yet reveals profound implications: the Earth is warming, but the sun’s output has not substantially increased. This observation underscores the critical role of greenhouse gases in climate change. The chemistry of these gases presents deeper reasons for this warming trend.
Carbon dioxide, the most commonly discussed greenhouse gas, arises primarily from the combustion of fossil fuels, deforestation, and various industrial processes. Its molecular structure consists of one carbon atom and two oxygen atoms. When CO2 is released into the atmosphere, it can remain there for centuries, long outlasting many of the other greenhouse gases. Its long atmospheric lifetime makes it particularly hazardous in terms of climate change, as even small increases in concentration can lead to significant warming.
Methane, in contrast, is a more potent greenhouse gas, albeit present in much lower concentrations. It comprises one carbon atom and four hydrogen atoms, and is produced primarily through livestock digestion, landfill decomposition, and natural gas extraction. Methane is vastly more effective at trapping heat than CO2, having a global warming potential (GWP) approximately 25 times greater than that of carbon dioxide over a century. However, methane has a shorter atmospheric lifetime, persisting for about a decade before it oxidizes into CO2 and other substances.
Nitrous oxide, another significant greenhouse gas, is released during agricultural practices and fossil fuel combustion. This gas, consisting of two nitrogen atoms and one oxygen atom, has a GWP nearly 300 times that of carbon dioxide. Nitrous oxide poses a dual environmental threat: it not only contributes to global warming but also plays a critical role in ozone layer depletion, thus exacerbating environmental issues.
Meanwhile, water vapor, the most abundant greenhouse gas, operates on a different trajectory. It is not directly emitted by human activities; rather, its concentration is influenced by the temperature of the atmosphere. Warmer air holds more moisture, leading to an increase in water vapor as temperatures rise due to climate change. This creates a feedback loop: warming leads to higher water vapor levels, which further traps heat and amplifies warming, an aspect that complicates climate change models.
While the chemistry of these gases elucidates the mechanisms by which heat is trapped in the atmosphere, it also raises profound ethical and existential questions. The intricate relationship between human activity and natural systems cannot be understated. The exponential rise in greenhouse gas concentrations since the Industrial Revolution correlates directly with industrialization, urbanization, and increased energy demands. This anthropogenic influence disrupts the natural balance, with ramifications that are all too evident in the present-day climate crisis.
Moreover, the interplay of these gases with earth’s ecosystems highlights a crucial point: climate change is not merely a scientific issue but a deeply interwoven tapestry of societal dynamics, economic interests, and ethical considerations. The quest for fossil fuel alternatives, carbon capture technologies, and sustainable practices underscores humanity’s urgent call to mitigate greenhouse gas emissions.
As the implications of climate change unfurl, it becomes vital to champion research and advocacy. A thorough understanding of the chemistry behind climate change empowers individuals and communities to make informed decisions appreciating the interconnectedness of their actions and the larger environmental context. Furthermore, comprehensive policies aimed at reducing greenhouse gas emissions are imperative to reverse the current trajectory of warming.
Thus, understanding the chemistry of climate change — how gases trap heat — reveals crucial insights into our fragile existence on this planet. Through respectful stewardship of our environment, we can endeavor to mitigate these alarming trends and preserve Earth for future generations. Acknowledging the foundational chemistry influencing our climate is the first step toward sustainable solutions and a reconciliatory relationship with nature.