As we navigate the treacherous waters of climate change, a question emerges: Could acid rain make a resurgence as a consequence of global warming? The specter of acid deposition, once thought to have been significantly mitigated by stringent regulations, might loom ominously on the horizon. Understanding this phenomenon requires delving into the intricate interplay of environmental factors that contribute to its formation and prevalence.
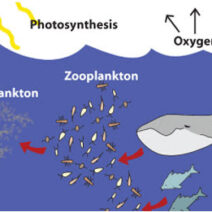
Firstly, it’s essential to grasp what acid rain is. Primarily a result of sulfur dioxide (SO2) and nitrogen oxides (NOx) being released into the atmosphere, these compounds can originate from various sources, including industrial emissions and motor vehicle exhaust. Once released, they undergo complex chemical reactions in the atmosphere, often facilitated by the presence of water vapor, ultimately leading to precipitation that carries these acids back to the Earth’s surface.
Historically, the United States and Europe witnessed the devastating effects of acid rain, particularly in the late 20th century. Ecosystems were irrevocably altered, aquatic life suffered, and human structures, particularly those composed of limestone and marble, eroded under the corrosive effects of acid deposition. Yet, a determined global response led to substantial reductions in sulfur and nitrogen emissions, offering a glimmer of hope. But is that hope now tinged with uncertainty?
Enter global warming—a phenomenon that influences myriad environmental factors, including weather patterns, precipitation, and the overall atmospheric composition. As the planet warms, the implications for acid rain become increasingly perplexing. Warmer temperatures can exacerbate the formation of acid rain by influencing the chemical reactions that convert SO2 and NOx into their more hazardous forms. Elevated temperatures encourage increased evaporation and, consequently, can lead to more water vapor in the atmosphere. This excess moisture can act as a catalyst for acid formation, leading to more acidic precipitation.
Moreover, changes in precipitation patterns are expected to accompany global warming. Regions that experience intense storms may witness increased acid rain due to a higher volume of precipitation carrying these contaminants to the ground. Conversely, areas facing drought may experience concentrated pollutants, which can remain in the atmosphere longer and contribute further to acid deposition when rainfall does occur. The challenge lies in predicting where and how these changes will manifest.
Consider the potential effects on rural and urban landscapes alike. Forests, particularly those situated in mountainous regions, are susceptible to acid rain, which can leach essential nutrients from the soil, thereby weakening trees and making them more vulnerable to diseases and pests. Urban areas, with their concrete and metal structures, could see accelerated deterioration, leading to increased maintenance costs and safety hazards. How then do we prepare for a future where acid rain could re-emerge as a significant environmental threat?
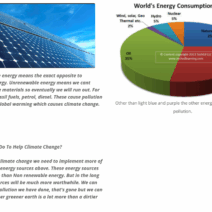
Addressing the potential resurgence of acid rain requires a multifaceted approach. Innovations in technology must be prioritized. For instance, advancements in energy production can lead to cleaner technologies that reduce SO2 and NOx emissions. Transitioning to renewable energy sources—such as wind, solar, and hydroelectric systems—can dramatically lower the release of harmful pollutants, lessening the risk of acid rain.
Furthermore, regulatory frameworks must be robust and adaptable. Ensuring that emission standards remain stringent in light of changing climatic conditions is crucial. Collaborative international initiatives could forge pathways to harmonize these regulations, fostering a unified front against pollutants that affect not just individual nations, but the global community. The task may seem daunting, but it’s essential for safeguarding ecosystems and public health.
Education and public awareness also play pivotal roles in combating the potential return of acid rain. Communities must be informed about the consequences of their actions concerning pollution. Initiatives aimed at promoting sustainable practices—such as reducing vehicle emissions, utilizing public transport, and improving energy efficiency—can significantly mitigate acid-forming pollutants. When individuals understand their contributions to acid rain, they may be more inclined to adopt environmentally friendly behaviors.
Moreover, ongoing research is vital to understand the complex dynamics of global warming and acid rain better. Scientists must investigate the changes in atmospheric chemistry brought about by climate shifts and how these changes affect not only the formation of acid rain but also the broader ecological implications. Real-time monitoring of air and soil quality can provide invaluable data, allowing for timely interventions to reduce the impact of acid deposition.
Ultimately, the question of whether acid rain will return is not one of inevitability but rather of action—the choices we make now will define the environmental landscape of the future. As global temperatures rise and atmospheric conditions shift, the risk of acid deposition looms larger. However, through concerted efforts in technology, regulation, education, and research, society can confront this potential challenge head-on. The time for action is now; the stakes are too high to ignore.