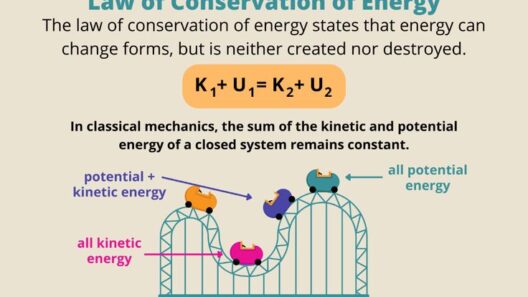
The Law of Conservation of Energy is a cornerstone principle in the realm of physics, positing that energy cannot be created or destroyed, only transformed from one form to another. Yet, a perplexing question lingers: who first elucidated this fundamental concept? Engaging with the history of this law reveals a tapestry interwoven with the contributions of several brilliant minds over centuries—a collective endeavor culminating in the scientific understanding we possess today.
Inquisitive Beginnings: The Notion of Energy
To comprehend the origins of the Law of Conservation of Energy, one must first appreciate how the concept of energy itself evolved. The term “energy” entered the scientific lexicon in the early 19th century, derived from the Greek word “energeia,” meaning “activity” or “operation.” Prior to this, the understanding of forces and motion were often relegated to physical phenomena observed in nature. Early philosophers such as Aristotle contemplated the nature of motion, but their musings lacked the empirical rigor that characterized modern science.
However, the roots of the law can be traced back to the work of notable physicists like Galileo Galilei and Isaac Newton. Galileo’s investigations into projectile motion, along with Newton’s formulation of the laws of motion and universal gravitation, laid the groundwork for understanding dynamics. They implicitly suggested that motion could be quantified and that energy existed in various forms, each influencing the behavior of physical systems. Yet, the explicit articulation that energy is neither gained nor lost was still on the horizon.
The Spark: Chemical and Mechanical Insights
As the 19th century unfurled, the Industrial Revolution catalyzed profound technological advancements, prompting scientists to deepen their exploration of energy transformations. Notably, the work of James Prescott Joule provided pivotal empirical evidence for the conservation principle. In one of his renowned experiments, Joule demonstrated the conversion of mechanical work into heat by mechanically agitating water and measuring temperature changes. His findings underscored a crucial insight: energy may change forms, but the total amount remains invariant—a radical departure from earlier notions of energy as fluid or universal.
Simultaneously, the burgeoning field of thermodynamics emerged, bolstered by the contributions of physicists such as Rudolf Clausius and William Thomson (Lord Kelvin). Clausius, in particular, formulated the first and second laws of thermodynamics, which codified the energy conservation principle within the realm of heat transfer and entropy. These revelations were not merely academic; they had significant implications for engineering, allowing for the optimization of engines and establishing a quantifiable framework for thermal processes.
Unity in Diversity: The Collaborative Efforts
The journey toward conceptualizing the Law of Conservation of Energy was not the province of a single individual. Instead, it was the product of collaboration and discourse among brilliant minds. Figures such as Hermann von Helmholtz further advanced this discourse in the mid-19th century. Helmholtz articulated the principle more broadly by integrating various forms of energy—kinetic, potential, thermal—into a unified theory. He announced the conservation of energy principle as a potent declaration of how interconnected and dynamic the universe is, where systems are in constant flux yet adhere to certain invariants.
This sentiment echoes throughout disciplines far beyond the confines of physics. The concept has resonated profoundly within chemistry, biology, and even economics, establishing energy as a fundamental entity that pervades all aspects of existence. It raises an important contemplation: in our modern world, where energy consumption and sustainability are pressing issues, how can we apply this fundamental law to drive innovations in energy efficiency and renewable resources?
Modern-Day Reverberations: An Energy-Conscious Future
As we stand at the crossroads of ecological crises and technological advancements, the Law of Conservation of Energy compels us to reconsider our relationship with energy. With the burgeoning awareness of climate change consequences, there lies a pressing challenge for contemporary society: can we harness our understanding of energy transformations to foster sustainable practices that respect this fundamental law?
Innovations such as solar energy, wind power, and advancements in energy storage technologies reflect a consciousness rooted in the very essence of the conservation principle. These technologies not only exhibit various transformations of energy forms but also highlight the importance of efficiency and responsible consumption. Ultimately, the Law of Conservation of Energy does not only remain an academic pursuit; it serves as a beacon illuminating the path toward a sustainable future, urging us to innovate and evolve in harmony with natural laws.
In conclusion, tracing the origins of the Law of Conservation of Energy reveals a rich tapestry of scientific inquiry. From early philosophical musings to rigorous experimentation and interdisciplinary collaboration, this principle emerged as one of the linchpins of modern science. As stewards of the Earth, we find ourselves challenged to apply the lessons of energy conservation to create a more sustainable and conscious society. How will we respond to this challenge, and in what ways will we revolutionize our energy practices in pursuit of a harmonious coexistence with our planet?