The examination of closed systems raises an intriguing question: are they perpetually energy conserving? The inquiry spans across various scientific disciplines, particularly thermodynamics, physics, and environmental science. At the heart of this exploration lies the fundamental principle of energy conservation and the intricacies involved in different types of systems—both open and closed.
To embark on this examination, it’s crucial to define what constitutes a closed system. In thermodynamics, a closed system is a physical system that does not exchange matter with its surroundings but can exchange energy. This distinction leads us to the first major point of discussion: the nature of energy transfer within these systems. While closed systems can conserve energy over time, they do not inherently do so; this conservation depends on variables such as temperature, pressure, and energy states.
One of the most pivotal concepts to consider is the First Law of Thermodynamics, which stipulates that energy cannot be created or destroyed, only transformed from one form to another. In a closed system, energy transformations may manifest through work and heat exchange. For example, in a perfect closed system, the total energy remains constant, but the energy can transfer between potential and kinetic forms. However, when we introduce real-world conditions, imperfections arise, leading to energy dissipation in the form of heat or friction. Thus, practical closed systems may not achieve absolute energy conservation.
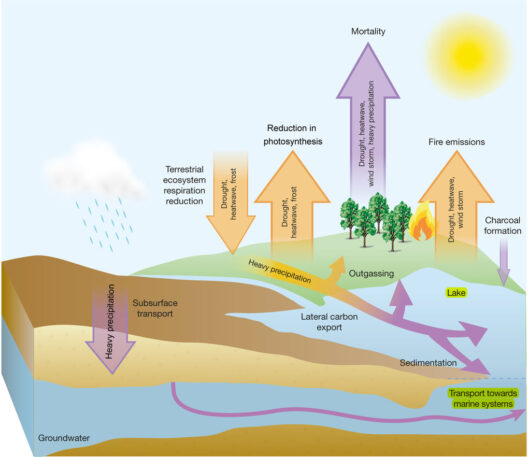
Another fascinating aspect of closed systems is their application in various fields, including ecosystems and industrial processes. In ecosystems, closed models may describe nutrient cycles where matter is recycled, yet energy from the sun enters the system continually, necessitating an open system perspective for complete understanding. Similarly, in thermodynamic processes, engines contribute to the discussion. The efficiency of engines illustrates that while they operate under closed conditions, energy losses are unavoidable due to irreversible processes like combustion inefficiencies and mechanical friction.
Furthermore, the second law of thermodynamics introduces an important layer to our understanding. It asserts that in natural processes, the total entropy of a closed system can never decrease. This principle implies that while energy can be conserved in an ideal closed system, practical scenarios exhibit a trend toward disorder or waste heat. This entropy factor is critical when considering energy conservation over time, as it suggests that while energy is conserved in quantity, it may not be conserved in quality; usable energy becomes increasingly less effective.
Investigating the implications of energy conservation in closed systems leads to environmental considerations. One primary concern is the sustainability of these systems. Industrial closed-loop systems, designed to minimize waste and utilize resources efficiently, strive for energy conservation. Yet, even in such systems, input energy is affected by external factors, including resource depletion and ecological impact. The quest for a truly sustainable closed system raises questions regarding the long-term viability of energy conservation strategies.
The intricacies of closed systems expand even further when exploring the concept of dynamic equilibrium. In chemical reactions, for instance, if a reaction achieves equilibrium within a closed system, the energy transfer may reach a standstill, implying that while the energy within the system is conserved, the system is not actively utilizing energy for further reactions. Such scenarios can mislead one into thinking that energy conservation equates to stasis and stability, while in reality, systems are often in a state of flux until external influences alter their trajectory.
Moreover, closed systems are not exclusively characterized without input or output alterations. The introduction of feedback loops can significantly influence the conservation of energy. In ecological models, feedback can lead to self-regulating behavior that either supports or undermines energy conservation. For instance, a closed agricultural ecosystem that effectively recycles nutrients can lead to greater energy efficiency. Conversely, an inefficient loop can precipitate energy drains, reducing the system’s overall effectiveness.
Another consideration when questioning the nature of closed systems is the technological advancements that allow for improved energy efficiency. Innovations in materials, engineering, and design have enabled the development of systems that approach ideals of closed systems. For instance, advancements in renewable energy technologies aim to create energy systems that can potentially optimize energy conservation even within ostensibly closed structures. However, it’s essential to recognize that these advancements are contingent on ongoing energy input from external resources, thus blurring the lines of the closed system classification.
As society progresses towards sustainable practices, the examination of closed systems becomes increasingly pertinent. The integration of renewable energy sources into closed systems appears to challenge the notion of absolute energy conservation. Given that these systems draw on external energy inputs, they exemplify the increasingly complex nature of systems that straddle the definitions of open and closed.
In conclusion, while closed systems can be designed to conserve energy, they are not always energy-conserving in practice. The intricacies of energy transfer, entropy, and feedback dynamics complicate the ideal of a perfectly closed energy-conserving system. Real-world applications reveal that energy conservation is influenced by a multitude of factors, some of which may lead to a loss of efficiency and effectiveness. Understanding these nuances encourages deeper contemplation about sustainable practices and the interaction between systems and their environments, challenging us to rethink energy consumption and conservation in a multifaceted world.