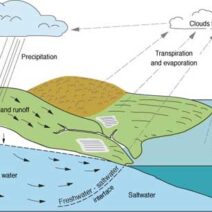
Energy conservation is a fundamental principle in physics, often articulated as the law of conservation of energy. This law posits that energy cannot be created or destroyed; it can only change forms. However, the interplay between different types of energy—especially kinetic energy—raises intriguing questions about whether total energy can truly be conserved in the absence of kinetic energy. This discourse delves into that observation, examining underlying principles that illuminate the complexities of energy conservation.
First, it is imperative to delineate the types of energy pertinent to this discussion. Energy exists in various forms, including kinetic energy (the energy of motion), potential energy (energy stored in an object due to its position), thermal energy (related to temperature), and several others. When evaluating systems where kinetic energy is diminished or non-existent, one must consider whether the total energy amount remains unchanged. The conundrum arises: If kinetic energy is zero, can we still assert that total energy is conserved?
To answer this question, one must explore the conservation of energy within isolated systems. In classical mechanics, the total mechanical energy (the sum of kinetic and potential energy) remains constant provided no external work is done on the system. For example, consider a pendulum. At the apex of its swing, kinetic energy is at a minimum (zero at the peak) while potential energy is maximized. As it descends, kinetic energy increases at the expense of potential energy. Throughout this motion, however, the total mechanical energy remains conserved, showcasing that while kinetic energy may fluctuate, it does not negate the conservation of total energy.
However, upon examining systems where kinetic energy is absent, such as inunmoving physical structures or thermal equilibrium, the conservation law still holds. In such scenarios, potential energy can transform into other forms, such as thermal energy—illustrating that energy conversion can occur even in the absence of kinetic energy. Thus, while kinetic energy may not always be present, total energy can still be conserved through potential or thermal transformations.
Moreover, the realm of thermodynamics introduces another layer of complexity. The first law of thermodynamics reinforces the principle of energy conservation but highlights conversion efficiency and heat transfer. In thermal systems, energy can transform into heat, which inherently carries no kinetic component. For instance, in an ideal gas, changes in temperature (and therefore thermal energy) do not necessitate movement, emphasizing that conservation principles operate beyond merely kinetic energy considerations.
Nonetheless, certain systems may display conditions where it seems as though total energy conservation falters. Take, for instance, systems undergoing friction. When an object slides across a surface, kinetic energy transforms into thermal energy due to frictional forces. Here, one could question if the energy is “lost.” However, what occurs is not energy loss but energy redistribution; the total energy remains conserved within the confines of the system, albeit in different forms. Therefore, even when kinetic energy dissipates through friction, total energy remains intact.
Another striking example lies within the realm of quantum mechanics, where energy conservation nuances become intricate. Quantum systems occasionally exhibit non-intuitive behavior, where particles may seem to violate classical energy conservation principles—but not in the totality of the system. The Heisenberg Uncertainty Principle, for instance, allows for “borrowed” energy during short timescales. Though observations may suggest dissonance in energy conservation, once the larger quantum system is accounted for, the principle holds true across observable phenomena.
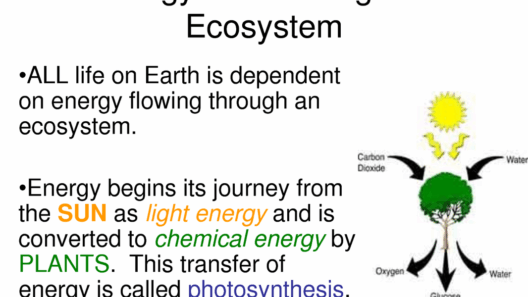
Furthermore, energy storage and transformation in ecosystems serve as a testament to the conservation principle. In photosynthesis, for example, plants convert solar energy into chemical energy through metabolic processes. Although no kinetic energy is involved in this conversion process, the total energy remains conserved, fundamentally underpinning the energy transfer throughout an ecosystem. This highlights the significance of energy transitions rather than a mere focus on kinetic energy.
Exploring the broader implications of energy conservation further fosters appreciation for its ubiquitous influence. From mechanical devices to biological systems, energy transformation maintains equilibrium—shaping physical laws, environmental sustainability, and the future of technological advancement. The human endeavors to harness and utilize various energy forms, especially renewable resources, emphasize this interaction’s importance, recognizing the interdependence of energy forms.
In conclusion, the question of whether total energy can be conserved if kinetic energy isn’t present ultimately affirms the law of conservation of energy across distinct contexts. While kinetic energy plays a crucial role in many systems, it is not the sole determiner of total energy conservation. Instead, energy can transition from one form to another, demonstrating resilience regardless of the individual components at play. Understandably, this complexity captivates intellectual inquiry, revealing a tapestry of relationships fundamental to the universe’s operation. Appreciating these intricate dynamics will enrich one’s understanding of both physical principles and ecological sustainability, fostering a deeper awareness of our energy-dependent existence.