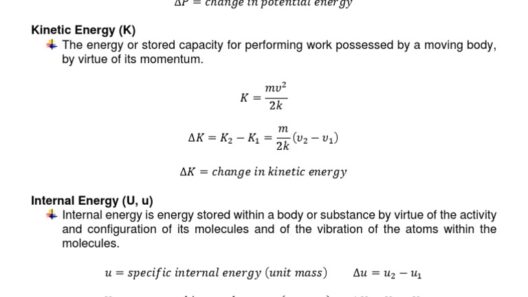
The principle of conservation of energy is a fundamental tenet in physics, asserting that energy cannot be created or destroyed, only transformed from one form to another. When considering the question of whether any process violates the law of conservation of energy if run backward, one must engage in an exploration of both thermodynamic principles and philosophical implications. This inquiry delves into concepts such as reversibility, entropy, and the nuances of energy systems.
To approach this question, it is essential to first understand what is meant by “running a process backwards.” In physical terms, this denotes a sequence of events that proceeds in the opposite chronological order. For example, consider a process such as a ball rolling down a hill. Running this process backward would require the ball to ascend the hill autonomously. In real-world scenarios, such inversions often seem implausible due to the inherent laws governing energy transformations.
One of the cornerstone principles underpinning this discourse is the second law of thermodynamics. This law incorporates the concept of entropy, which quantifies the degree of disorder within a system. Entropy tends to increase over time in isolated systems, meaning that as energy transforms, some portion becomes increasingly unavailable for work. Therefore, when a process is reversed, the natural progression toward increased entropy would suggest a contradiction with energy conservation. For instance, if one were to see ice spontaneously melting into water and then reforming into ice without any external energy input, it would defy this fundamental law.
Thermodynamic systems can be categorized into reversible and irreversible processes. Reversible processes are hypothetical constructs where systems can return to their original states without any net change in the surrounding environment. In contrast, irreversible processes result in increased entropy and are characterized by energy dispersal that renders the system unable to return to its initial state without outside intervention. When analyzing whether reversing a process would violate energy conservation, one must acknowledge that while energy is conserved in both situations, the *available* energy for doing work is depleted in irreversible scenarios.
Understanding the difference between potential energy and kinetic energy is crucial in the context of these concepts. When a ball rolls downhill, it converts potential energy into kinetic energy. If the process were to be run backward—restoring kinetic energy into potential energy—this would necessitate an external force acting upon the system, thereby challenging any notions of spontaneity. In essence, while energy itself remains conserved, the practical interfaces with real-world phenomena complicate matters significantly.
In addition to mechanical processes, chemical reactions also illuminate the discussions surrounding energy conservation. Certain chemical transformations are exothermic, releasing energy that can be harnessed for other work. If these reactions were to be reversed, one would require equivalent energy input to facilitate the reverse reaction, thereby adhering to conservation laws. Nonetheless, this emphasis on energy inputs leads to questions about efficiency and energy losses in practical applications, underscoring that conservation is often not just about existence but also about utility.
Real-world examples abound and serve to illustrate these principles clearly. Take the example of an automobile engine. Fuel combustion within an engine releases energy that is transformed into mechanical work. Should the process run backward, it would imply an engine that self-combusts without energy input—an absurd scenario that underscores the second law of thermodynamics and conservation of energy. Realistically, reversing such chemical processes necessitates external energy sources, reinforcing the idea that while energy itself is conserved, usable energy degrades through inefficiencies.
The implications of energy conservation extend beyond physical systems and provoke epistemological considerations. The idea of time’s arrow, a concept in physics, speaks to unidirectional progression—implying that processes have an orientation that aligns with the increase of entropy over time. This inherent directionality challenges the concept of reversing processes in a meaningful way. Philosophically, one might ponder whether an understanding of time itself is intrinsically linked to the capacity for energy transformations and reversibility.
Furthermore, advanced topics in contemporary physics delve into scenarios such as quantum mechanics, where the notion of reversibility appears more complex. Some interpretations of quantum theory suggest that quantum states can be “reversed” under certain conditions, a concept that may not conform neatly to classical notions of energy consistency. This intricacy signifies a frontier in understanding energy conservation and the potential for phenomena that appear to transgress established laws, yet still remain firmly rooted in conservation principles at a fundamental level.
In conclusion, the question of whether any process violates the principle of conservation of energy when run backward prompts significant exploration into the nature of energy itself and the laws that govern it. While energy remains conserved across all transformations, practical scenarios—particularly those subject to the second law of thermodynamics—render most backward processes an impracticality requiring external energy or inputs. This intricate interplay between energetic processes, entropy, and philosophical inquiries presents a fertile ground for continued exploration, ensuring that the dialogue on conservation remains vibrant and essential in the context of environmental responsibility and sustainable practices.