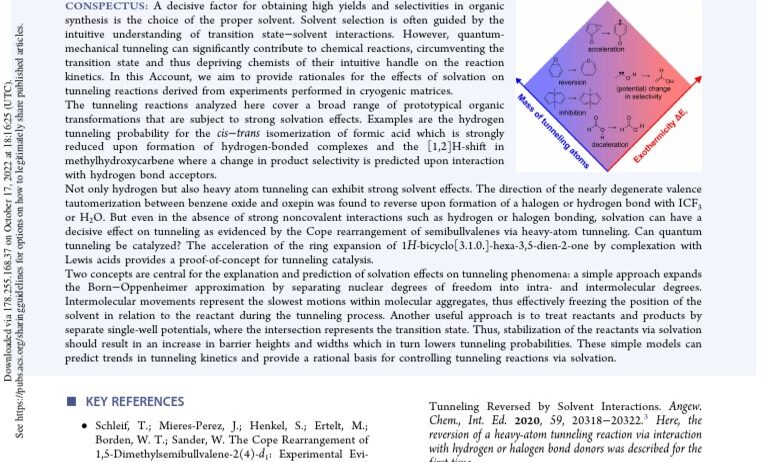
At the intersection of quantum mechanics and chemistry lies a phenomenon both baffling and captivating: quantum tunneling. This enigmatic effect allows particles to traverse energy barriers that, by classical physics standards, they should not surpass. The question arises: does this quantum behavior in chemical reactions lead to energy conservation or a net expenditure of energy? To unravel this mystery, one must delve into the subtleties of quantum mechanics and the nature of chemical reactions.
At its core, quantum tunneling can be likened to a bird that flits across an imposing mountain range rather than flying over it. The mountain represents an energy barrier, one that classically would require significant energy input to overcome. Yet, in the quantum realm, particles such as electrons have the capacity to ‘tunnel’ through these barriers, emerging on the other side with an unsettlingly casual air. This observation challenges our traditional understanding of energy dynamics and conservation.
In chemical reactions, energy conservation hinges upon the principle that energy cannot be created or destroyed, only transformed. When molecules collide, they exchange energy, yielding products that differ in stability and energy content. Typically, the activation energy—a threshold that reactants must overcome to form products—must be met to facilitate this transformation. However, quantum tunneling introduces an unconventional twist to this energetic ballet.
Consider the familiar landscape of a hill. If molecules colliding in a reaction represent balls on this hill, they typically require sufficient kinetic energy to roll over the peak. In contrast, quantum tunneling allows these balls to evaporate momentarily, passing through the dense fabric of space-time, emerging on the other side with little to no energy spent overcoming the hill. This effortless traversal raises the question of energy exchange: is energy being conserved, or is it simply masked by the quantum mechanics at play?
To better understand the implications of quantum tunneling on energy conservation, it is essential to explore its role in various chemical reactions. For instance, in enzymatic reactions, the activation energy barrier can be significantly lowered by the presence of an enzyme, enabling faster reaction rates. In systems where quantum tunneling plays a pivotal role, such as proton transfer in biochemical processes, the activation energy can effectively drop to near-zero levels. This leads to a scenario where reactants can access lower energy states without an energy input commensurate with traditional expectations.
In exploring how quantum tunneling operates within chemical reactions, it becomes clear that the overall energy balance may remain consistent, while the pathways to achieve these transformations become remarkably diverse. If we examine proton transfer as a specific example, the kind that occurs in acid-base chemistry, we realize that quantum tunneling allows protons to shift between reactive sites in a fraction of the time that classical models would predict. In effect, this behavior does not violate energy conservation; rather, it adds an additional layer of complexity to the energy landscape, underscoring the importance of quantum considerations.
Energy conservation in quantum tunneling can be particularly significant in the context of environmental chemistry. Consider the cycle of energy exchange in natural processes. When we think about the role of plants in carbon fixation, their photosynthetic machinery operates not merely on mechanical energy transfer but also incorporates quantum coherence and tunneling. This enables plants to harness the sun’s energy with remarkable efficiency as electrons traverse molecular barriers, ultimately producing energy-rich glucose. Thus, quantum tunneling can enhance our understanding of how these systems operate, providing insights into energy conservation in biogeochemical cycles.
Nonetheless, a caveat accompanies the fascination of quantum tunneling: the complexity it introduces may lead to misconceptions about energy expenditure in chemical processes. While the act of tunneling can circumvent energy barriers, other factors, such as thermal fluctuations and environmental conditions, ultimately play a crucial role in determining the success of these quantum phenomena. It is vital not to romanticize quantum tunneling as a panacea; instead, it exists within a grand tapestry interwoven with myriad influences that shape the direction of chemical reaction pathways.
Moving forward, the intrigue of quantum tunneling presents both challenges and opportunities for scientific inquiry. The revelation that quantum effects can influence energy dynamics in chemical reactions may enhance our understanding of reaction mechanisms, thereby aiding in the development of new materials or the optimization of existing chemical processes. Moreover, by harnessing tunneling behaviors in chemical systems that could lower energy requirements, we may potentially design more sustainable reactions and enhance energy conservation in various applications.
In conclusion, quantum tunneling serves as a fascinating bridge linking the abstract world of quantum mechanics with the tangible reality of chemical reactions. While it challenges established notions of energy conservation, it simultaneously opens avenues for deeper insights into the relationship between energy, reaction rates, and molecular interactions. The journey through this quantum landscape not only reveals the elegance of nature’s design but also highlights the urgent need for innovation in energy systems, especially in our quest for sustainability amidst the growing threat of climate change. Embracing the principles of quantum tunneling may very well lead to transformative approaches that align with the ideals of energy efficiency and ecological responsibility.