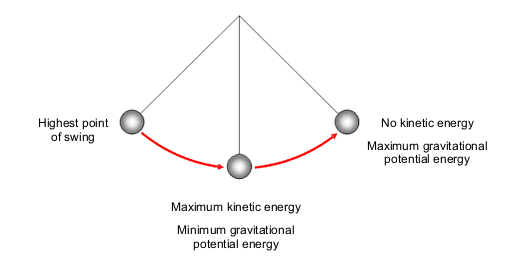
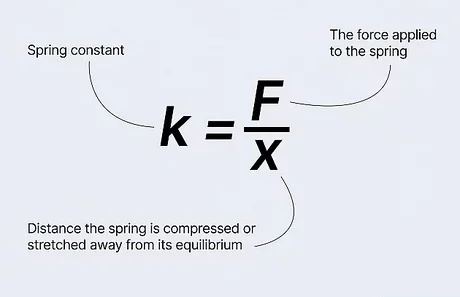
The Law of Conservation of Energy is a fundamental principle in physics, asserting that energy cannot be created or destroyed; it can only be converted from one form to another. While this concept is often associated with mechanical systems, it also intricately applies to thermal energy and temperature. Temperature, as a measure of the average kinetic energy of particles, invites exploration into how energy transformations underpin thermal phenomena. Understanding the relationship between the Law of Conservation of Energy and temperature challenges conventional perceptions and reveals profound implications for environmental dynamics.
At its essence, the concept of temperature serves as a macroscopic manifestation of energy at the microscopic level. In essence, temperature quantifies the kinetic energy of molecules within a substance. When substances absorb heat, their molecular vibrations intensify, and consequently, their temperature rises. This relationship between energy and temperature epitomizes the Law of Conservation of Energy: as energy transfers into a system, its temperature correlates with the energy introduced. For instance, when you heat water on a stove, the addition of thermal energy directly elevates its temperature, transforming it from a liquid state toward boiling.
One might ponder, then: what happens to energy when temperature levels change? Under the umbrella of the Law of Conservation of Energy, when energy is added to a system—like heating a kettle—the overall energy content of the system augments. However, not all energy conversion is straightforward. Some energy dissipates as heat due to friction or imperfect insulation, illustrating an essential nuance of this law. This aspect highlights that while energy itself is conserved, the distribution and efficiency of energy transformations can vary significantly.
In the realm of thermodynamics, we encounter a deeper exploration of energy conservation via the laws delineating thermal systems. The zeroth law establishes thermal equilibrium, while the first law, also known as the law of energy conservation, encapsulates the transformative processes of energy in thermal systems. This understanding becomes pivotal when dissecting phenomena like phase changes—when water transitions to steam, energy is required for this transformation. Interestingly, during phase changes, temperature remains constant despite energy input, epitomizing the crucial distinction between energy and temperature that is essential in broader contextualizing.
This distinction extends into various natural processes. Consider the Earth’s climate system: it acts as an intricate interplay of energy flows between the sun, the atmosphere, oceans, and land surfaces. The energy absorbed from the sun fundamentally alters the temperature and, consequently, the state of water in various forms—ice, liquid, vapor. When considering climate change, this conservation principle underscores how energy imbalance occurs. An increase in greenhouse gases traps more heat in the atmosphere, augmenting surface temperatures. The excess energy does not disappear; rather, it reshapes climatic patterns, further affecting ecosystems and biodiversity.
Moreover, the implications of the Law of Conservation of Energy reach into man-made systems. Devices reliant on energy transformation, such as engines or solar panels, epitomize this law at work. Understanding that energy is conserved can enhance the efficiency of energy use. For instance, when designing a solar panel system, comprehending the conversion efficiency from sunlight into electricity leads to optimizations that can significantly affect operational costs and power generation. Similarly, improving insulation within buildings reduces energy loss, thereby minimizing overall consumption—a direct application of conserving thermal energy.
Shifting perspectives on energy through the lens of temperature not only bears scientific importance but also fosters a philosophical regard for our environment. As temperatures rise globally, understanding the conservation of energy illuminates the need for sustainable practices. Each entity and system must optimize energy management to mitigate negative impacts on the environment. A systematized approach that respects the conservation law allows for innovative solutions in renewable energy, pollution reduction, and resource management. Creativity exists at the intersection of understanding energy laws and addressing climate challenges.
An engaging realization derives from exploring how the transition of energy is not homogenously distributed. The Second Law of Thermodynamics underscores this, introducing the notion of entropy in thermal systems. While energy is conserved, it often degrades in useful forms, leading to increased disorder. As energy is transformed, some energy tends to dissipate into less useful forms, showcasing a dynamic balance within systems striving for equilibrium. This reality reinforces the need to work toward higher efficiency in energy usage, particularly in contexts like industrial applications and urban development.
As we contemplate these principles, the intersection of energy conservation and temperature naturally leads us to consider innovation rooted in ecological and sustainable paradigms. Emerging technologies, whether carbon capture or advanced thermal systems, align with the need for efficient energy use while respecting natural laws. Ultimately, understanding the Law of Conservation of Energy in relation to temperature challenges us to think sustainably—in every energy input, the output must be judiciously evaluated, ensuring resilient systems capable of sustaining life on this planet.
In summary, the Law of Conservation of Energy applies robustly to temperature, enriching our perceptions of thermal dynamics in both natural and artificial systems. Recognizing the intricate relationship between energy transformations and temperature can spark curiosity, inspire innovation, and ultimately position society on a more informed path toward addressing climate change and fostering ecological balance.