Have you ever pondered the magic behind a simple puddle disappearing on a warm day? What happens when water transitions from liquid to vapor? This phenomenon, known as evaporation, is not only intriguing but also reveals profound concepts in thermodynamics and conservation laws that govern our physical world. In this exploration, we will delve into how mass and energy are conserved during the evaporation process, weaving through science with an informative narrative.
To understand the conservation of mass and energy during evaporation, one must first grasp the process itself. Evaporation occurs when water molecules gain sufficient kinetic energy to overcome the attractive forces binding them in a liquid state, transitioning them into a gaseous state. This transformation is significant because it illustrates the interplay of molecular motion, energy exchange, and conservation principles.
At the crux of this discussion lies the law of conservation of mass, which states that mass cannot be created or destroyed in an isolated system. During evaporation, the amount of water retains its mass, even as it changes form. While the liquid water diminishes, the vapor produced is simply another manifestation of that very mass, now dispersed into the atmosphere. When considering a cup of water left unattended, one might intuitively accept that the water volume decreases. However, upon careful measurement, one would find that the mass—albeit in a different form—remains constant. This transitions us into a fascinating realm: where did the mass go? The water hasn’t vanished; it has merely transformed into vapor that is less visible but very much existent.
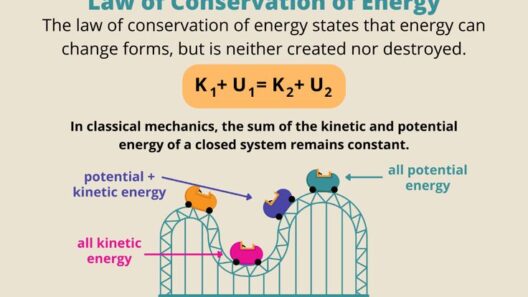
Equally remarkable is the conservation of energy, encapsulated in the law of conservation of energy. This principle asserts that energy cannot be created or destroyed, only converted from one form to another. During evaporation, thermal energy from the surrounding environment transfers to the liquid water molecules. This energy influx is crucial as it facilitates the molecular movement necessary for overcoming the cohesive forces that hold the water molecules together. The concept can be illustrated with a playful analogy: consider water as a group of dancers at a party. If nobody leaves the dance floor (the cohesive forces), the party continues, but once some dancers absorb enough energy from the music (thermal energy), they break away and start dancing solo (vaporizing). The party isn’t losing dancers; they are simply transitioning to a different form of expression.
Interestingly, evaporation also exemplifies the first law of thermodynamics, which states that the total energy of an isolated system remains constant. The heat energy used to energize the water molecules to phase transition gets equalized by a cooling effect in the remaining water. Thus, when a puddle evaporates, it cools the surrounding temperature, demonstrating an energy exchange in action. This leads to an intriguing challenge: can we harness this cooling effect for practical applications in reducing urban heat? This type of energy transfer has fundamental implications for microclimatic conditions in urban environments. The more we understand energy conservation in natural processes like evaporation, the better we can strategize in devising sustainable technologies.
Moreover, this dynamic interplay of mass and energy conservation is profoundly relevant to climate science. As global temperatures rise, the rates of evaporation increase, contributing to changes in weather patterns and the hydrologic cycle. The amount of water vapor in the atmosphere directly influences climate systems, affecting precipitation and temperature regulations. Thus, understanding the efficiency of this natural phenomenon can aid in crafting solutions to mitigate climate change.
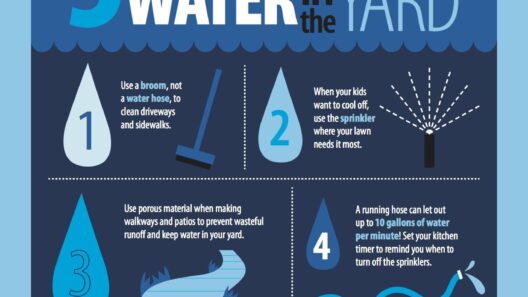
Another important aspect to consider is the role of surface area and temperature on the rates of evaporation. A larger surface area allows more molecules to escape at once, while higher temperatures provide more energy for molecular excitement. This is why, on a hot day, small puddles evaporate much faster than large bodies of water. The conservation principles remain unaltered, yet the dynamics change based on environmental conditions. An exploration into these variables invites further inquiry: how might urban development strategies capitalize on this understanding to maximize water efficiency and energy savings?
While we have highlighted the conservation of mass and energy in the process of evaporation, we must also acknowledge that practical implications extend far beyond a simple glass of water. The principles governing evaporation can inform us about weather systems, climate change, and even conservation techniques that can be implemented at an individual or communal level. Are we employing these principles to educate communities about resource management? Understanding how mass and energy interplay within our ecosystems contributes to the larger narrative of sustainable living and environmental stewardship.
In conclusion, the evaporation of water encapsulates fundamental scientific principles that govern our environment. The conservation of mass and energy during this phenomenon showcases a delicate balance that is paramount to the stability of our ecosystems. The interplay of molecular motion, energy transitions, and environmental factors presents an insightful exploration into the world of thermodynamics, directly linking to broader implications in climate science. As we challenge ourselves to delve deeper into these concepts, we embark on a path toward innovating solutions that resonate with our mission to protect and preserve the natural world.