The Law of Conservation of Energy, a cornerstone of physics, posits that energy cannot be created or destroyed; it can only change forms. This principle is akin to a philosophical reflection on existence: energy, much like life itself, is eternal but perpetually transforms. The question arises, however, whether this law can be broken or violated. In this exploration, we delve into the nuances of energy conservation, examining examples, implications, and the philosophical underpinnings of this profound assertion.
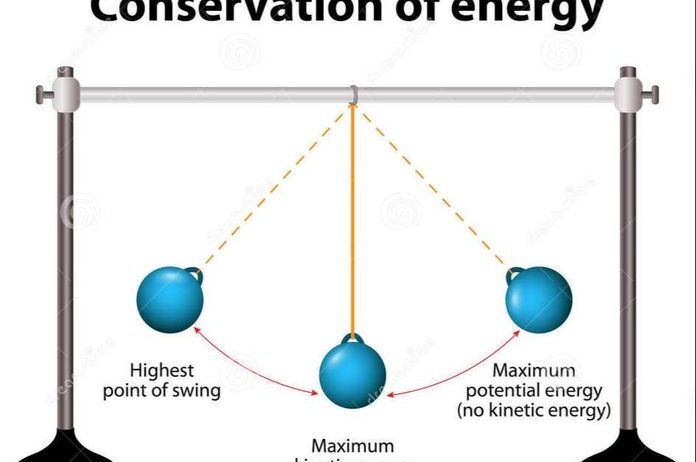
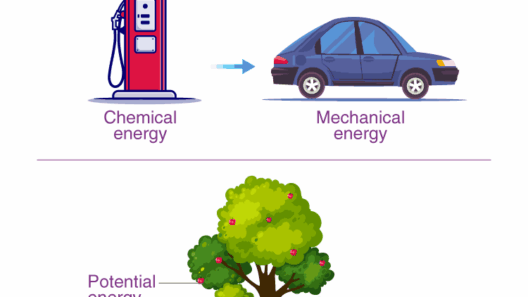
At its essence, the Law of Conservation of Energy asserts that the total energy in an isolated system remains constant. This law encompasses various energy forms, including kinetic, potential, thermal, and chemical energies. Consider an automotive engine, which converts chemical energy from fuel into kinetic energy to propel motion. This transformation is seamless, yet it remains within the bounds of conservation. When the vehicle comes to a halt, kinetic energy dissipates into heat, yet no energy is lost; it merely changes form.
However, in applying these principles to real-world scenarios, we often encounter phenomena that seem to contravene this immutable law. Herein lies one of the law’s most intriguing aspects: while energy in a closed system remains constant, open systems interact with their environments in complex ways. For instance, take solar panels. They capture solar radiation, a form of energy from the sun, converting it into electrical energy. This process may appear as an energy arrival—almost like magic—but in reality, it is an intricate dance of energy transformation rather than creation.
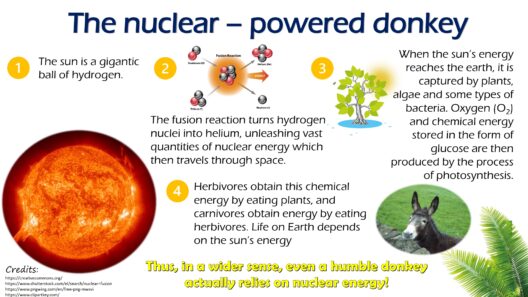
Another captivating example arises in the realm of nuclear fusion. When atomic nuclei combine to form larger nuclei, energy is released, seemingly from nowhere. While it appears as if new energy is birthed, what’s truly happening is a conversion of mass into energy, as articulated by Einstein’s famous equation, E=mc². This equation elucidates that mass and energy are interchangeable; therefore, energy is not created in a vacuum but emerges from the conversion of mass—a nuance that preserves the sanctity of conservation despite the dramatic appearance of energy gain.
Theoretical concepts further supplement these phenomena. Consider the notion of a perpetual motion machine, a device that could operate indefinitely without an external energy source. Such contraptions tantalize inventors and scientists alike, yet they consistently illustrate the fallacy of violating energy conservation laws. The second law of thermodynamics asserts that entropy, or disorder, in a closed system will never decrease; hence a perpetual motion machine, inherently producing more energy than it consumes, is impossible. These fantastical inventions serve as metaphors for unattainable dreams and remind us of the immutable structure that governs our universe.
The intersection of energy conservation and technology illustrates additional quandaries. Engineers seek to optimize energy efficiency, yet improving a system’s efficiency can paradoxically lead to increased energy consumption in a different context. Take, for example, the rise of energy-efficient household devices. While these appliances may use less energy operationally, their proliferation can lead to a net increase in energy usage due to heightened demand. This phenomenon is often referred to as the “rebound effect,” where efficiency gains inadvertently stimulate demand, illustrating the complexities and sometimes unintended consequences of our attempts to break free from energy constraints.
Environmental considerations further complicate our relationship with energy conservation. The burning of fossil fuels transforms stored chemical energy into kinetic energy, powering the modern world. However, this energy transformation generates greenhouse gases, introducing externalities that disturb the natural equilibrium. The challenge lies not in the violation of the conservation law, but in the mismanagement of energy transformation processes and their impact on ecological systems. This interplay between energy and the environment emphasizes our responsibility to harness energy in a manner that aligns with sustainable practices.
The philosophical implications of this discussion prompt a reevaluation of human intervention in natural energy flows. As humanity grapples with climate change and resource depletion, the question arises: How do we best utilize the finite energy resources available to us? Innovations in renewable energy sources, such as wind, solar, and hydropower, illustrate our capacity to work in harmony with natural energy cycles, rather than attempting to exploit or violate them. By aligning human ingenuity with natural laws, we create an energy ecosystem that respects the foundational principles of conservation.
In summary, the Law of Conservation of Energy stands as an indelible truth within the realms of physics and philosophy. While on the surface, some phenomena may seem to contravene this law, a deeper understanding of energy transformations reassures us that energy is, indeed, eternal but forever transmuting. Through our interactions with energy, we have the opportunity to engage positively with our environment, forging paths that honor conservation while exploring innovative energy solutions. In this intricate dance, it becomes evident: we are not merely users of energy, but custodians of a delicate balance that sustains our planet and civilization.