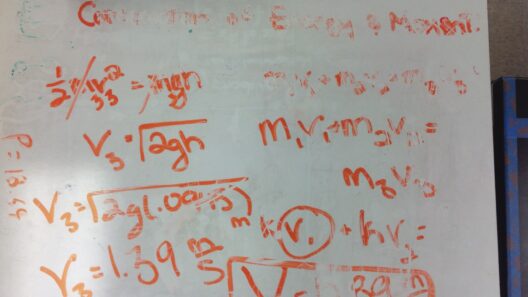How do we know that energy is conserved? This fundamental question resonates with both curious minds and seasoned scientists alike. The Law of Conservation of Energy asserts that the total energy in an isolated system remains constant; it can neither be created nor destroyed, only transformed from one form to another. But how do we empirically validate this law? Let’s embark on an exploration of the scientific principles and experimental evidence that underpin this pivotal concept.
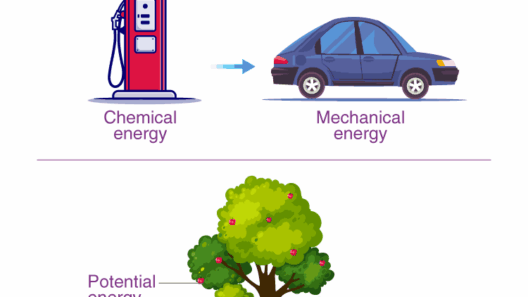
At the heart of physics lies the concept of energy. Energy manifests in various forms, including kinetic, potential, thermal, and chemical energy, among others. In any physical process, energy shifts from one form to another—think of a roller coaster. As the coaster ascends a hill, kinetic energy diminishes while potential energy accumulates. When it plunges down, that potential energy converts back into kinetic energy, propelling the coaster forth. But where does the energy go? Do we simply take it on faith that no energy mysteriously disappears? The meticulous observations made by scientists over centuries support this hypothesis.
The first significant validation of energy conservation comes from classical mechanics. Sir Isaac Newton’s work laid the groundwork through analysis of forces and motion. Newton’s laws demonstrate consistency in energy transformations, showing that the sum of potential and kinetic energy remains constant in a closed system. For example, consider a pendulum. As it swings from one side to another, energy shifts between potential energy at the highest points and kinetic energy at the lowest. Remarkably, if air resistance were negligible, the energy would keep oscillating, maintaining its total amount.
Yet, classical mechanics only scratches the surface. Delving deeper, we arrive at thermodynamics, which expands our understanding to heat energy. The First Law of Thermodynamics, which aligns closely with the Law of Conservation of Energy, states that when energy is added to a system, it can either increase the system’s internal energy or do work on its surroundings. In essence, energy is a currency used in nature, meticulously accounted for. Heat engines, like those in automobiles, illustrate this principle effectively. In these systems, chemical energy from fuel is converted into mechanical energy and thermal energy, all while obeying conservation laws.
Next, we traverse the realm of modern physics, exploring the microscopic domain. The advent of quantum mechanics introduced new complexities regarding energy conservation. At the quantum level, particles are governed by probabilistic behaviors, yet energy conservation remains a steadfast principle. The famous double-slit experiment, for instance, vividly demonstrates the wave-particle duality and highlights energy conservation even amid quantum uncertainty. Energy conservation in quantum mechanics is further reinforced through various particle interactions and decay processes, where the energy before and after remains invariant.
As we delve into electromagnetism, we encounter another manifestation of energy conservation. James Clerk Maxwell’s equations describe how electric and magnetic fields interact, demonstrating energy conservation through electromagnetic waves. For instance, when a charged particle accelerates, it emits electromagnetic radiation, resulting in energy loss from the particle. Nevertheless, this energy must be conserved and can instead manifest in the radiated waves, proving the interconnected fabric of energy transformations. Thus, through various branches of physics, fundamental energy conservation is underscored across multiple arenas.
However, energy conservation is not devoid of challenges. An interesting inquiry arises: what happens in scenarios involving non-isolated systems? Take, for instance, the infamous phenomenon of energy dissipation due to friction. In such cases, mechanical energy is not lost but transformed into thermal energy, raising the question of whether all energy transformations can indeed be accounted for. Here, the law is upheld, albeit in more complex forms where energy remains conserved but is merely converted into less useful forms. This aspect highlights the importance of understanding energy’s quality, not merely its quantity.
We also grapple with the implications of energy conservation on contemporary environmental challenges. Acknowledging that energy cannot be created or destroyed emphasizes the need for sustainable energy practices. The transition to renewable energy sources is an imperative response to the growing climate crisis. Solar, wind, and hydroelectric power represent transformations of natural energy into usable forms, showcasing nature’s inherent ability to conserve and provide energy in a sustainable manner. By leveraging these sources, we can uphold the Law of Conservation of Energy while addressing climate change and minimizing environmental degradation.
In conclusion, the Law of Conservation of Energy stands as a cornerstone of scientific inquiry, supported by an array of evidence from classical mechanics to modern physics. Energy undergoes transformation rather than termination, a concept that has profound implications not just for academic pursuits but also for how society approaches energy consumption. While challenges exist, such as energy dissipation and the upstream dynamics of non-isolated systems, the overarching principle remains intact: energy is conserved. Each exploration into this law unveils the intricate tapestry of our universe, making the study of energy both a joyful pursuit and a critical dialogue in the quest for a sustainable future.