Calorimetry, a branch of thermodynamics, is the science of measuring heat transfer in physical and chemical processes. It plays a pivotal role in various scientific disciplines, ranging from chemistry to environmental science. Its relevance to the conservation of energy is profound, as it provides insight into the principles governing energy transfer, transformation, and conservation in our ecosystems and beyond.
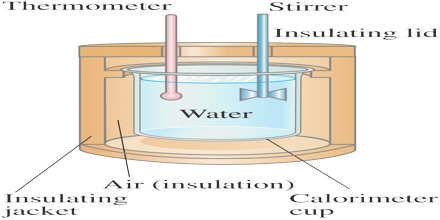
To grasp the relationship between calorimetry and energy conservation, one must first delineate what calorimetry entails. At its core, it involves determining the heat involved in reactions or physical changes. The most commonly employed instruments in this field are calorimeters, which capture heat exchanges by maintaining a controlled environment. When a chemical reaction occurs, the calorimeter measures the resultant temperature change, thus allowing scientists to quantify the energy released or absorbed during the process. This empirical measurement is foundational in understanding broader energy dynamics.
The principle of conservation of energy, articulated in the first law of thermodynamics, posits that energy cannot be created or destroyed, only transformed from one form to another. Calorimetry embodies this principle by providing a method to observe transformations in energy. For instance, in combustion reactions, the chemical energy stored in fuel is converted to heat energy. Through calorimetric analysis, one can deduce the efficiency of this transformation, gauging how much of the energy is actually harnessed for productive use—not lost as waste heat.
One common observation in calorimetry relates to the marked energy changes observed during phase transitions. Consider the process of melting ice. When heat is applied, the temperature remains constant until the solid ice transitions into liquid water. This phenomenon—the latent heat of fusion—highlights a critical aspect of thermodynamics: energy is expended in breaking intermolecular bonds rather than in increasing temperature. Understanding such transitions is crucial in nature, where ecosystems are highly sensitive to thermal energy dynamics.
Whether in natural ecosystems or engineered systems, the implications of calorimetric studies are crucial when addressing sustainability and conservation. For instance, the efficiency of renewable energy systems, such as solar panels and biofuels, can be precisely evaluated through calorimetric methods. By measuring the energy output against input and losses, stakeholders can optimize these systems for better performance, thereby enhancing their viability as sustainable alternatives to fossil fuels.
Furthermore, calorimetry shines a spotlight on anthropogenic effects on energy conservation. The modern industrial era has brought forth significant changes in energy use and conservation. By analyzing energy consumption patterns, calorimetric assessments inform conservation strategies aimed at reducing our ecological footprint. For example, energy utilized in heating systems or energy-intensive industrial processes may be meticulously quantified and subsequently adjusted. Such measures can lead to substantial energy savings—a vital endeavor in the quest to mitigate climate change and conserve natural resources.
This leads to a reflection on environmental processes, where calorimetry serves as a diagnostic tool to unravel the intricate interactions among species, energy transfer in food webs, and the stability of ecosystems. In biology, calorimetry can reveal how organisms adapt their metabolic processes in response to thermal environments. It demonstrates that even the smallest temperature changes can significantly influence organismal behavior and survival, emphasizing the importance of energy conservation in ecological systems.
Upon delving deeper into the implications of calorimetry, one recognizes its significance in climate modeling—an essential component for predicting future scenarios under varying climatic conditions. Accurate calorimetric measurements contribute to developing models that simulate energy interactions between the atmosphere, oceans, and terrestrial ecosystems. These models are instrumental in informing policy decisions regarding climate action, showcasing the long-term benefits of energy conservation efforts in mitigating global warming.
In the context of energy consumption in households, calorimetric principles can be applied to promote energy-efficient practices. Understanding the energy savings derived from using insulated cooking appliances versus traditional methods can lead to more sustainable lifestyle choices. Additionally, public awareness campaigns that educate the masses on the significance of calorimetry can foster a culture of energy conservation, thereby enhancing community engagement in climate initiatives.
As one navigates through the art and science of calorimetry, it becomes apparent that a deeper appreciation for energy conservation is not merely an academic exercise but rather, a fundamental necessity in the contemporary era. Calorimetry reveals the often-overlooked nuances of energy interactions, encouraging a mindset that prioritizes sustainability and stewardship of our planet.
In conclusion, calorimetry serves as a bridge between the quantitative assessments of heat transfer and qualitative understandings of energy conservation. It elucidates the pivotal role of energy transformations in both natural and engineered systems. The meticulous measurement and analysis provided by calorimetric studies empower us to devise strategies to optimize energy use. In confronting the myriad challenges associated with climate change, embracing the principles of calorimetry paves the way for informed action and sustainable practices that protect our environment. Hence, recognizing and implementing the insights derived from calorimetry is essential for fostering a resilient and energy-conscious society.