The principle of conservation of energy is a fundamental concept in physics that asserts that energy cannot be created or destroyed, only transformed from one form to another. When applied to a closed system, this principle elucidates the intricacies of energy dynamics and illuminates the mechanisms through which energy is conserved over time. Understanding how energy conservation operates within closed systems necessitates a clear delineation of what constitutes a closed system, the various forms of energy at play, and the implications of energy transformation within such systems.
A closed system is defined as a physical system that does not exchange matter with its surroundings, although energy can be exchanged. This concept is pivotal in both theoretical and applied physics, as it enables scientists and engineers to model behaviors and predict outcomes within defined parameters. Examples of closed systems include insulated thermodynamic systems, planetary systems, and specific mechanical constructions like pendulum systems. The encapsulation of matter within boundaries facilitates a controlled environment where energy transfers can be observed and analyzed.
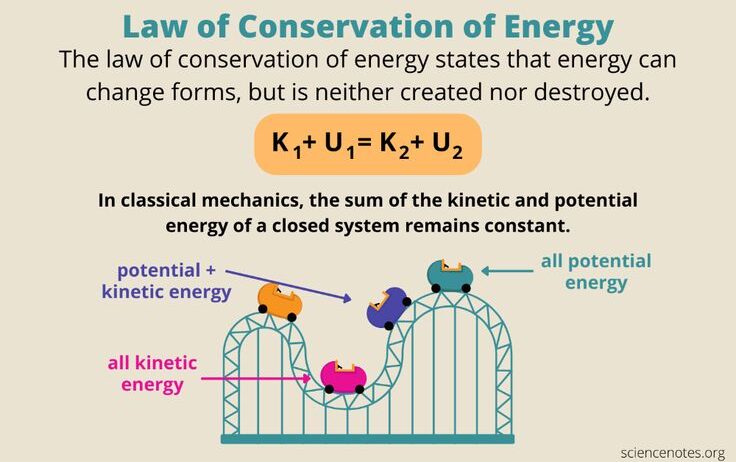
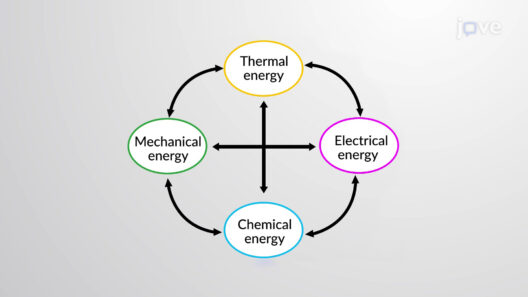
At the core of energy conservation is the recognition that energy exists in various forms, primarily kinetic energy, potential energy, thermal energy, electromagnetic energy, and chemical energy. Kinetic energy is the energy associated with the motion of objects, proportional to the mass of the object and the square of its velocity. Potential energy, on the other hand, is energy stored within an object due to its position or configuration. For instance, when lifting an object against gravity, work is done, and the energy transferred to the object becomes gravitational potential energy. This interplay between kinetic and potential energy is illustrated by the motion of a pendulum, where energy continuously transforms between the two forms, yet the total mechanical energy remains constant in an ideal closed system without friction.
The transformation of energy is subject to the laws of thermodynamics. The first law of thermodynamics, often referred to as the law of energy conservation, states that the total energy of an isolated system remains constant. By applying this law to a closed system, one can analyze the energy transfers that occur during transformations. For example, in a closed container with gas, when the gas is compressed, its potential energy increases, and thermodynamic principles suggest that there will be a corresponding increase in temperature as the kinetic energy of gas molecules escalates due to compression.
Moreover, closed systems possess the propensity to establish equilibrium states. When energy enters or exits a system, the various energy forms strive to reach a state of balance. A classic illustration of this principle can be observed in a closed system containing a liquid and an evaporating surface. As the liquid evaporates, its molecules absorb thermal energy, demonstrating the transformation from liquid to gas, while the energy balances out when the vapor reaches a certain saturation point. This dynamic balance results in a stable environment where the energy distribution remains consistent over time.
Understanding closed systems also leads to insights in various practical applications, including engineering, environmental science, and climatology. Engineers utilize the principles of energy conservation to design systems for optimal efficiency. Take, for instance, the design of cars and buildings, where minimizing energy loss through high-efficiency engines and insulated structures enhances overall energy conservation. Utilizing renewable energy sources within these systems can further augment the conservation of energy by harnessing energy transformations from solar or wind power, minimizing reliance on conventional fossil fuels.
In an environmental context, the conservation of energy is seminal in addressing climate change. The closed system of the Earth operates under the laws of thermodynamics, with solar energy being the primary input. Understanding how energy is transformed within Earth’s climate system helps in predicting climatic behavior and formulating mitigation strategies. This highlights the importance of reducing greenhouse gas emissions, which enhance the interplay of energy within the closed system of Earth’s atmosphere. A plethora of energy conservation strategies, such as improving energy efficiency and adopting sustainable practices, can curb excessive energy consumption and its consequential impact on climate dynamics.
Furthermore, the application of energy conservation principles within social systems, such as communities and economies, reflects the broad relevance of this topic. As we strive for sustainability, understanding that energy conservation resonates through every facet of daily life, from household energy efficiency to large-scale industrial processes, becomes increasingly significant. By fostering an understanding of these concepts within communities, societies can mobilize collective efforts toward achieving energy conservation goals and advocating for policies that promote sustainability.
In conclusion, the principles of conservation of energy apply significantly to closed systems, providing invaluable insights into the behavior of energy within defined boundaries. Understanding the transformations between kinetic, potential, and other forms of energy elucidates the dynamics that govern closed systems. Recognizing these principles not only propels advancements in technology and engineering but also underscores the urgency of addressing climate change and achieving sustainability through informed practices. The interplay of energy conservation and ecological integrity is essential for fostering a harmonious relationship between human activities and the planet’s health, ultimately contributing to a more sustainable future.