Energy conservation in chemical reactions is a fundamental principle governed by the laws of thermodynamics. At its core, the energy transformation during these reactions provides insight into the intricate dynamics of matter. Understanding the nuance involved in these transformations, from the smallest atoms to complex molecules, offers a new perspective on both the micro and macro scales of chemical processes. This discourse invites an exploration into how energy conservation operates at various stages of chemical interactions.
First and foremost, it is essential to recognize that atoms are the basic building blocks of matter. Each element consists of atoms that have unique properties, governed by the arrangement of protons, neutrons, and electrons. When we examine the energy associated with these atoms, we delve into the concept of potential energy, which can be released or absorbed during chemical reactions. This potential energy is often stored within the bonds between atoms; when a bond is formed or broken, energy changes hands, which is central to the notion of conservation.
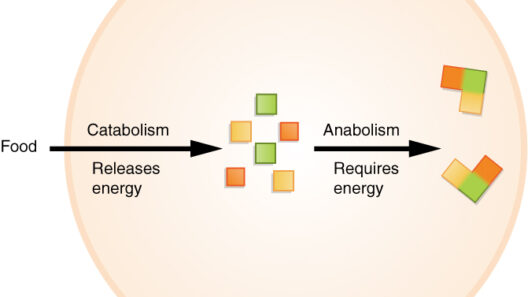
The first law of thermodynamics, often referred to as the law of energy conservation, asserts that energy cannot be created or destroyed, only transformed from one form to another. In chemical reactions, we see energy transitioning between kinetic and potential forms. When reactants collide, the energy of motion (kinetic energy) can lead to the breaking of bonds, allowing atoms to rearrange and form new substances. Conversely, when new bonds are created, energy is released, usually in the form of heat or light. This exchange is a prime example of how energy is conserved in the context of chemical processes.
As reactions progress, they can be categorized as either exothermic or endothermic. Exothermic reactions release energy, resulting in a net decrease in the system’s internal energy, while endothermic reactions absorb energy, leading to an increase in internal energy. Take combustion as an illustrative example: a fire consumes fuel and emits heat and light, encapsulating the exothermic reaction paradigm. In contrast, photosynthesis epitomizes an endothermic process, where plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen, illustrating energy’s pivotal role in biochemical transformations.
The allure of these reactions lies not merely in their energy exchanges but in the stoichiometry that governs them. The coefficients in a balanced chemical equation reveal the proportional relationships between reactants and products. This quantitative aspect emphasizes that for every action—every bond formed or broken—there exists a reaction that conserves energy overall. Understanding these ratios allows chemists to predict the energies involved in reactions with remarkable precision.
Moreover, to delve deeper into the molecular realm, consider the concept of activation energy. This is the threshold energy required to initiate a reaction. The role of catalysts in lowering activation energy showcases another dimension of energy conservation. Through alternative pathways, these catalysts allow reactions to proceed with less energy input, effectively promoting efficient energy usage throughout the process. This angle underscores the remarkable adaptability of chemical reactions in conserving energy, revealing opportunities for innovation, particularly in sustainable practices.
The significance of energy conservation extends beyond the lab, influencing broader environmental considerations. In many chemical processes, such as those involved in industrial manufacturing or energy production, understanding how to manage energy efficiently can drastically reduce carbon footprints. Institutions now look to mimic natural processes, implementing bio-inspired designs that adhere to the principles observed in nature. By harnessing the power of energy transformation and promoting chemical efficiency, we can further push towards a sustainable future.
The intricate dance of energy within chemical reactions fundamentally alters our interpretations of natural processes. As we observe various reactions within ecosystems, we see how energy conservation not only sustains life but also influences ecological balance. Plants convert solar energy into chemical energy, sustaining the food web, while decomposers salvage energy during decay, returning nutrients to the soil, thus continuing the cycle of life. This interconnectedness highlights the importance of conserving energy at every stage of chemical transformation, as each small act resonates throughout the ecological abyss.
In conclusion, exploring how energy is conserved in chemical reactions—from atoms to molecules—unveils a mesmerizing narrative of transformation and sustainability. Within this framework, we find opportunities for innovation and the responsibility to protect our planet. By perpetuating energy efficiency and understanding the delicate balance of chemical interactions, we can hope to inspire collective action towards combating climate change. The elegance of these reactions serves as a reminder of the power of chemistry: as we unravel the complexities of nature, we are also equipped to forge a path toward a more sustainable future.