Have you ever wondered how energy flows and transforms around us? Imagine a seemingly endless cycle where nothing disappears, only changes form. This phenomenon is known as the Law of Conservation of Energy, a principle fundamental to understanding the interactions in our universe. The challenge, however, lies in demonstrating this law in a tangible and engaging way. Below, an exploration of methods to illustrate this principle, making science accessible and enjoyable for all.
The Law of Conservation of Energy asserts that energy cannot be created or destroyed; it can only be converted from one form to another. This principle is not merely theoretical; it underlies countless processes in the environment, technology, and our daily lives. But how can we layman’s illustrate such an abstract concept? Here are some fascinating experiments and phenomena that not only demonstrate this law, but also captivate the imagination.
The Pendulum Swing
First, let’s consider the classic pendulum. Suspend a weight (usually a small ball) from a fixed point with a length of string. Pull the weight back and release it. As it swings, observe the transformation of energy. At the highest point, the pendulum possesses maximum potential energy. As it descends, this potential energy converts into kinetic energy, reaching maximum speed at the lowest point of the swing. As it ascends on the other side, kinetic energy transforms back into potential energy.
The playful question to ponder here is: What would happen if you introduced friction, say from air resistance or a rough string? Would the pendulum eventually stop swinging? This scenario demonstrates another critical element of energy transformation: energy losses, usually in the form of heat. Through this simple setup, one can observe the conversion of energy forms while acknowledging that energy is always conserved, albeit with some losses along the way.
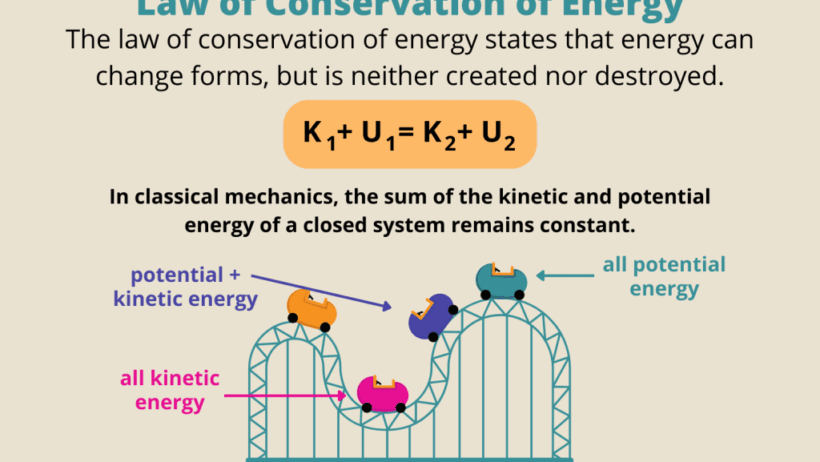
The Roller Coaster Effect
For those seeking a more exhilarating approach, consider the roller coaster as a prime example of energy transformation. Envision a roller coaster climbing to the apex of a hill. At this moment, the train is brimming with potential energy. As it plummets downwards, this energy morphs into kinetic energy, propelling the coaster forward. Each rise and fall illustrates the continuous interplay between potential and kinetic energy.
A challenge arises when we think about design: How can engineers optimize a roller coaster to maximize excitement while adhering to energy conservation principles? What safety measures need to be in place? This inquiry leads to a deeper appreciation of how energy transformation principles are applied in engineering and physics, emphasizing the delicate balance required to ensure both thrill and safety.
Chemical Reactions
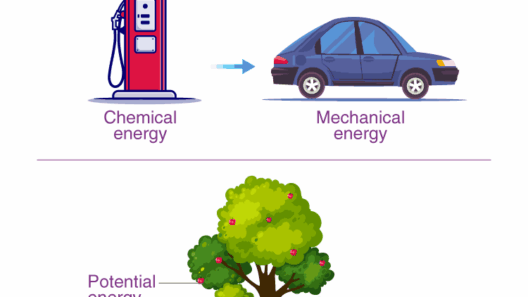
Energy transformation is not confined to mechanical systems; it is also evident in chemical reactions. Take, for instance, the exothermic reaction of combustion. When a fuel like wood or gasoline burns, it releases energy in the form of heat and light. This energy originates from the chemical bonds broken during the reaction. Similarly, in endothermic reactions, such as photosynthesis, plants absorb energy (typically from sunlight) to transform carbon dioxide and water into glucose and oxygen. Here, one can observe the conservation of energy as it transitions from light energy to chemical energy.
The challenge is to conceive how we utilize these energy transformations in daily life. What might our world look like without a basic understanding of chemical energy? Consider, for instance, renewable energy sources like solar panels that harness light energy, converting it into usable electrical energy. Such explorations encourage critical thinking about our current energy consumption and the implications for our planet.
The Heat Dynamics of Cooking
Another everyday demonstration of the Law of Conservation of Energy occurs in the kitchen. When cooking, energy transfer is at play. When heat is applied to food, thermal energy transfers to the molecules within it, transforming their state and flavor. Whether boiling water or baking bread, there is a seamless transition from electrical or gas energy into thermal energy, demonstrating conservation in action.
This prompts a playful question about energy waste: How much energy is lost during cooking, and can we minimize it? Exploring more efficient cooking methods or energy sources can be a compelling challenge. Can we cook a meal with less energy while maintaining or improving its quality? Addressing such concerns ties the principle of energy conservation to real-world applications, informing sustainable practices.
Conclusion
Ultimately, the Law of Conservation of Energy invites curiosity and exploration. Whether through intriguing experiments like pendulum swings, the thrilling dynamics of roller coasters, the transformation in chemical reactions, or even the practical energy dynamics observed in cooking, each scenario enriches our understanding of energy. Emphasizing that while energy is always conserved, inefficiencies will manifest—our societal challenge becomes clear: how to harness energy effectively while fostering innovation that benefits both humanity and the environment.
In recap, embracing the exploration of energy transformations offers profound insights into the natural world. Through engaging demonstrations and mindful challenges, we can not only grasp an essential scientific principle but also advocate for responsible energy use in our lives to address pressing issues like climate change. The journey of energy and its conservation is foundational to our existence; understanding it is pivotal to ensuring a sustainable future.