Energy is everywhere, pervading every facet of our physical universe. The concept of conservation of energy posits a foundational principle: in a closed system, energy cannot be created or destroyed; it merely transforms from one form to another. This article delves into the scientific methodologies for substantiating this pivotal principle, arousing curiosity and shifting perspectives on how we perceive energy in our daily lives.
To prove conservation of energy, one must approach the subject through a myriad of scientific experiments and historical contexts. These include both classical mechanics and thermodynamics. Let us explore several methods to affirm this theorem, illustrating its significance in various disciplines of physics.
The Mechanical Perspective
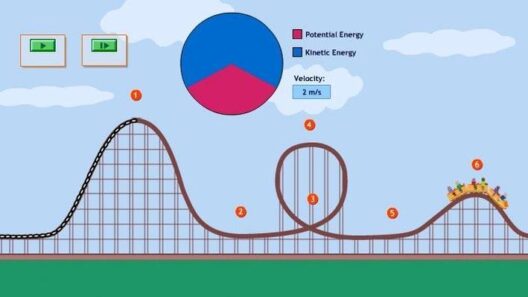
One of the quintessential methods for demonstrating the conservation of mechanical energy involves pendulum experiments. The pendulum, a simple yet profound system, oscillates due to gravity and inertia. As it swings, the energy interchanges between kinetic and potential forms. At the apex of its swing, potential energy reaches its zenith while kinetic energy wanes to zero. Conversely, at the lowest point of the swing, kinetic energy is maximized, and potential energy diminishes.
To quantitatively explore this phenomenon, one can employ the equations of motion. For example, the gravitational potential energy (U) can be expressed as U = mgh, where ‘m’ is mass, ‘g’ is the gravitational acceleration, and ‘h’ is the height above a reference point. Kinetic energy (K), on the other hand, is defined as K = (1/2)mv², where ‘v’ is velocity. Consequently, by measuring the height and speed of the pendulum at various points during its swing, one can construct a clear illustration of energy transformation, verifying that the total energy remains constant, barring frictional forces.
Thermodynamic Investigations
Beyond mechanical systems, thermodynamics provides an alternative lens through which one can understand energy conservation. The first law of thermodynamics, also known as the law of energy conservation, asserts that the total change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
An effective experimental setup for illustrating this principle can be achieved using a calorimeter — an apparatus designed to measure heat transfer. By calorimetrically examining an exothermic or endothermic reaction, one can measure the heat absorbed or released, subsequently calculating work performed in the process. For instance, when burning a fuel, the heat evolved can be sensed as an increase in temperature of the surroundings, effectively transforming internal energy into thermal energy. The system, in accordance with the first law, ultimately proves that energy is conserved throughout the reaction.
The Role of the Law in Everyday Life
The implications of energy conservation extend far beyond theoretical physics; they permeate daily practices and technological advancements. In engineering, energy conservation principles guide the design of more efficient systems. Electrical generators, for instance, exemplify the transformation of mechanical energy into electrical energy with minimal loss, fundamentally relying on conservation principles to optimize performance.
Engineering applications, such as regenerative braking in electric vehicles, further demonstrate energy conservation’s practical significance. This system captures kinetic energy usually lost during braking, converting it back to electrical energy for re-use, embodying the essence of energy conservation in motion.
Advanced Systems: A Closer Examination
Exploring the nuances of modern physics, one may consider the realm of quantum mechanics and its relation to energy conservation. In quantum mechanics, energy conservation is unyielding, manifested in principles such as the conservation of quantum states. When particles interact, the total energy before and after the interaction is conserved, albeit invariably with complications posed by the uncertainty principle, which influences our comprehension of energy transactions at an atomic scale.
Experiments such as the double-slit experiment dramatically elucidate these concepts. By demonstrating how light (or matter) behaves like both waves and particles, this experiment opens a discourse on energy’s dual nature. Nevertheless, throughout all these interactions, the total energy remains consistent, thus reinforcing energy conservation’s unassailable legitimacy.
Conclusion: A Transformation of Understanding
Provable through countless experiments and observable in both mechanical and thermodynamic contexts, the conservation of energy remains a gateway to understanding the natural world. From simple pendulums to the intricate laws governing quantum particles, the principle serves as a robust framework within which scientists and engineers strive to innovate and improve. Engaging with the theme of energy conservation not only fosters a deeper appreciation for the laws governing existence but also incites a transformation in how we approach sustainability and conservation efforts in our daily lives.
By analyzing energy’s transformations and embracing the inevitability of conservation, individuals can cultivate a more profound respect for our planet’s finite resources, ultimately encouraging a shift towards responsible stewardship of energy. Thus, understanding energy conservation is not merely an academic pursuit; it forms the bedrock of a sustainable future.