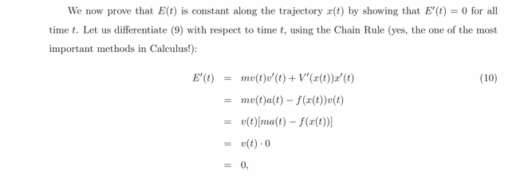
Understanding the law of conservation of energy is fundamental in both physics and our daily lives. This principle asserts that energy cannot be created or destroyed; rather, it can only change forms. This inexorable fact carries profound implications, spanning scientific fields as diverse as mechanics, thermodynamics, and even our own environmental practices.
At its core, the law of conservation of energy invites a deeper exploration into the nature of energy itself. To elucidate this concept, we will employ a step-by-step approach to prove the law using various methods, each illuminating the inescapable reality of energy transmutation.
Let us begin our journey with a thorough understanding of energy itself. Energy manifests in myriad forms: kinetic, potential, thermal, chemical, electrical, and nuclear, among others. The versatility of energy can often seem overwhelming, yet it is essential for initiating a critical examination of how it operates within closed systems.
Consider a simple pendulum. When raised to a height, it possesses potential energy due to its elevated position. Upon release, this potential energy transforms into kinetic energy as it swings downward. The maximum kinetic energy occurs at the lowest point of the swing, while the potential energy reaches its nadir. The transformation from potential to kinetic energy and vice versa exemplifies the conservation of energy in a tangible, observable manner.
Now, let’s quantify this conversion mathematically. The potential energy (PE) at the height (h) can be expressed as:
- PE = mgh
Where m is mass, g is the gravitational acceleration, and h is the height above the reference point. Conversely, the kinetic energy (KE) can be represented as:
- KE = ½ mv²
Here, v represents the velocity of the pendulum. At the apex of its trajectory, the energy is entirely potential. At the lowest point, energy transitions completely to kinetic form. The total mechanical energy (TME) in the system remains constant:
- TME = PE + KE = constant
This simple oscillatory motion illustrates not just a principle of physics, but also a broader truth: energy is continually at play, evolving and transitioning in forms.
Expanding upon this illustrative example, consider more complex systems such as a roller coaster. Initially, when the coaster is elevated to the top of a hill, it possesses substantial potential energy. As it descends, this energy is transformed into kinetic energy, powering the exhilarating rush of acceleration. Despite friction and potential losses due to air resistance, if we account for these factors, we can still observe a predominantly conserved energy principle. Notably, the work done against friction further underscores the types of energy transfer occurring within a system.
Now let’s delve into thermodynamic processes, which further reveal the law’s universal applicability. In a closed system, we can employ the first law of thermodynamics, often termed the law of energy conservation. This law states that the change in internal energy (ΔU) of a system equals the heat added to the system (Q) minus the work done by the system (W):
- ΔU = Q – W
This formula encapsulates a profound truth; it emphasizes that the transformation of energy into various forms is not purely mechanical. Instead, it applies broadly to heat and work interactions. In thermodynamic systems, even seemingly lost or wasted energy is accounted for in the energy balance, reinforcing the overarching principle of conservation.
Moreover, understanding energy conservation within ecological systems reveals an important dimension of applicability. When investigating energy flow through ecosystems, one can discern that energy from the sun is harnessed by plants through photosynthesis, converting solar energy into chemical energy stored in organic matter. As herbivores and carnivores consume this biomass, energy transfers through trophic levels, consistently reaffirming that energy always shifts from one entity to another—yet is never simply annihilated.
Exploring the implications of this theory raises crucial questions about our world—questions that challenge us to reconsider energy consumption patterns. In our daily lives, we often take energy for granted, failing to recognize its transformative capacities. The advent of renewable energy technologies, such as solar panels and wind turbines, encapsulates a shift towards sustainable practices, harnessing nature’s energy flow conservatively and efficiently. By utilizing energy wisely and seeking to minimize losses, we promote not just scientific understanding, but responsible stewardship of our planet.
In conclusion, the law of conservation of energy is a fundamentally essential principle that governs both natural phenomena and human activity. Through a myriad of examples—from pendulums to roller coasters, and even ecosystems—it is increasingly evident that while energy can shift and change forms, it is an ever-present and invariant aspect of our existence. Understanding this not only cultivates intellectual curiosity but also emboldens actionable insights towards sustainability and conscientious living. As we venture forth, maintaining an awareness of energy’s presence in all its forms will surely be pivotal in steering our future toward a more sustainable paradigm.