Calorimetry is a pivotal experimental technique used to measure the heat associated with chemical reactions or physical changes. Understanding whether energy is conserved during these processes is essential for various scientific applications. In this article, we will explore the principles of calorimetry, methodologies for conducting calorimetry tests, and key indicators to determine if energy conservation has occurred.
At its core, calorimetry involves the precise measurement of heat transfer. When a chemical reaction occurs, energy is either released or absorbed, significantly impacting the surrounding environment. The Law of Conservation of Energy states that energy cannot be created or destroyed; it can only change from one form to another. Thus, in a closed system, the energy released in an exothermic reaction should equal the energy absorbed in an endothermic reaction, allowing us to evaluate energy conservation effectively.
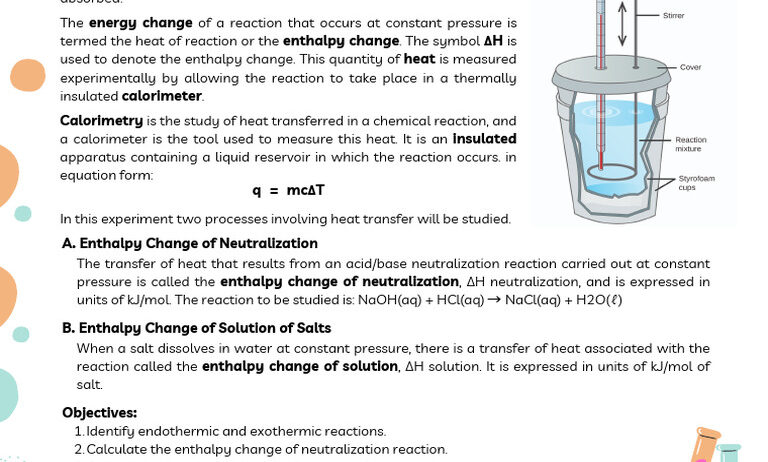
There are primarily two types of calorimeters utilized in experiments: the coffee cup calorimeter and the bomb calorimeter. The coffee cup calorimeter is a simple device often used in educational settings. It consists of two Styrofoam cups to minimize heat exchange with the external environment. The bomb calorimeter, on the other hand, is more sophisticated and is designed for measuring the heat of combustion of substances at constant volume. By familiarizing oneself with these apparatuses, one can understand how experimental conditions influence energy measurements.
The details surrounding the calorimetry test must be meticulously observed. To assess energy conservation in these tests, the first step is to establish a closed system where no heat can escape. This is crucial for accurate results. Any heat loss to the environment would lead to an incorrect interpretation of energy conservation. Hence, the insulation of the calorimeter is key.
Once proper equipment is assembled, initiate the calorimetry test by measuring the initial temperature of the reactants using a thermometer, which is crucial for baseline data. It serves as a starting point for calculating temperature changes during the reaction. This preliminary step will guide the subsequent assessments of heat flow and energy changes.
After the initial parameters are set, introduce the reactants to the calorimeter. It is imperative to monitor the temperature continuously. The ensuing temperature change reflects the amount of heat exchanged during the reaction. Utilize the formula q = mcΔT, where q represents heat energy (in joules), m denotes the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature. By applying this equation, one can quantify the energy released or absorbed, forming the basis for further analysis.
To ascertain whether energy was conserved, compare the calculated energy changes of the reactants and products. In a well-conducted calorimetry experiment, the total energy change of reactants should equal that of products. Thus, if a reaction produces an exothermic response, the energy lost by the reactants should precisely match the energy gained by the surrounding medium. Conversely, in an endothermic reaction, the system will absorb energy, which can be corroborated with the measured temperature rise of the calorimeter’s contents.
Another vital component to evaluate energy conservation is the calibration of the calorimeter itself. The calorimeter must be calibrated accurately before conducting tests. This involves using known amounts of energy to determine the heat capacity of the calorimeter. If the calorimeter is not calibrated correctly, erroneous data could lead to incorrect conclusions about energy conservation.
Precision in data collection cannot be overstated. Repeated trials are essential for deriving reliable results. Statistical analysis of the trials can help account for anomalies, leading to more credible insights. A consistent pattern will indicate energy conservation has occurred, whereas erratic results may suggest external factors are influencing the experiment.
Additionally, one must consider potential influences from the surrounding environment. Ambient temperature fluctuations or air currents can affect heat transfer, distorting the results. Conduct experiments with minimal external interference to ensure accurate readings. Furthermore, ensuring that the calorimeter remains fully insulated prevents any exchange of heat with the environment, bolstering the validity of the findings.
Interpreting the results of calorimetry tests also requires consideration of the chemical changes that occur. Some reactions may appear to conserve energy but could involve irreversible transformations. For instance, reactions that result in gas formation may lead to losses not accounted for in the calorimeter readings. Critical examination of reaction products is necessary to ascertain if they influence energy conservation dynamics.
In addition, external pressure changes can affect calorimetry tests, particularly in bomb calorimeters. It becomes essential to control for such variables and, if applicable, incorporate them into energy calculations. When reactions occur under non-standard conditions, corrections must be made to align findings with theoretical expectations.
Finally, adopting a holistic and critical approach to analyzing calorimetry results enhances understanding of energy conservation. Ultimately, a thorough investigation of all variables at play, rigorous experimental design, and robust data analysis can proficiently quantify energy changes in calorimetry tests. By vigilantly considering these aspects, one can confidently conclude whether energy conservation was upheld in their calorimetry experiments, contributing valuable insights for scientific advancement and applications in sustainability.