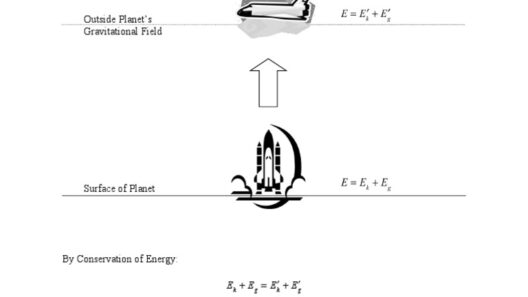
Understanding the conservation of energy law in thermodynamics is paramount for anyone curious about the principles of physics and their implications for real-world applications. At its core, the principle states that energy cannot be created or destroyed; rather, it can only change forms. This immutable law serves as a cornerstone in various scientific fields, including physics, chemistry, and environmental science. So, have you ever wondered what happens to the energy when you turn on a light bulb? That simple action spurs an intricate interplay of energy transformations that illustrate this fundamental law.
The conservation of energy can be observed through multiple phenomena. Consider the quintessential example of a pendulum: when swung, it converts potential energy at its highest point into kinetic energy at its lowest. Interestingly, this cycle repeats as long as external forces, like friction or air resistance, are minimal. It raises a compelling challenge—what if we could eliminate energy loss from our systems entirely? Could we engineer pendulums or other machines that perpetually operate without an external energy source?
Delving deeper, one must consider the various forms energy adopts. Energy manifests in kinetic, potential, thermal, chemical, electrical, and nuclear forms, among others. Each form plays a vital role in sustaining the functionality of our complex world. Kinetic energy, the energy of motion, is prevalent in vehicles, wind, and flowing water, while potential energy, stored energy based on an object’s position, can be easily visualized in a drawn bow or a massive dam holding back water. Recognizing these forms aids in grasping how different energy transformations occur in everyday scenarios.
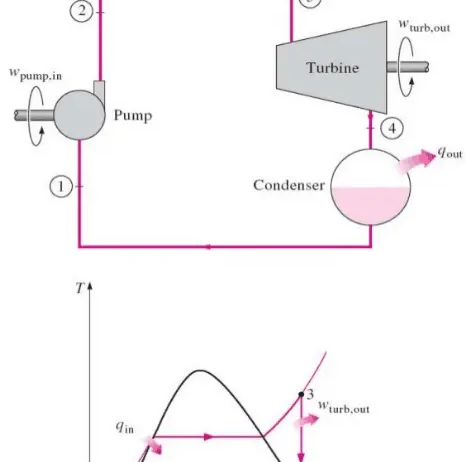
To exemplify, when a dam releases water, the potential energy is converted into kinetic energy as the water flows downward. In this case, energy is not lost; it merely transitions from one form to another, adhering to the conservation law. Thus, turbines harness this kinetic energy to generate electricity, further transforming energy into yet another form. This arrangement underscored the intricate dance of energy conservation that underlies technological advancements in renewable resources.
Another thought-provoking way to illustrate this principle involves examining how energy is used and manipulated in machines. Take, for instance, internal combustion engines in cars. Here, chemical energy stored in fuel undergoes conversion into thermal energy through combustion, subsequently becoming kinetic energy to propel the vehicle. However, efficiency is a critical consideration. A significant portion of that energy escapes as waste heat, presenting both an environmental and engineering quandary: how do we maximize efficiency while minimizing our carbon footprint? This conundrum emphasizes the importance of innovative technologies and renewable energy solutions.
A practical application of the conservation of energy law appears in the realm of conservation efforts. Understanding how energy is utilized in our homes and communities poses a challenge to modern society. If energy is conserved, how can individuals and industries reduce waste? It often begins with small actions: turning off lights when not needed, investing in energy-efficient appliances, and utilizing smart technology to monitor energy consumption.
Moreover, collective clean energy initiatives can foster substantial change. As a global community, shifting toward renewable resources such as solar, wind, and hydroelectric power addresses the sustainability challenge. By doing so, we not only preserve energy but also contribute to mitigating climate change—a pressing concern that affects all inhabitants of Earth.
Beyond energy conservation in practical applications, the theoretical implications are equally profound. Scientists and scholars continuously explore the frontiers of thermodynamic laws, endeavoring to unlock additional insights. The Second Law of Thermodynamics, for instance, introduces the concept of entropy, which stipulates that energy transformations inherently lead to some degradation of usable energy. This stark reality provides an excellent springboard for deeper inquiries: In a universe seemingly inclined towards chaos, how can we still strive for energy cohesion and conservation? A collaborative effort among thinkers, policymakers, and innovators becomes essential in addressing this pressing issue.
In summary, understanding the conservation of energy law in dynamics is a multifaceted endeavor, encompassing practical applications, theoretical principles, and societal implications. The law’s simplicity belies its complexity in actual practice, where energy continuously morphs into different forms. To meet the challenges presented by energy conservation, individuals must adopt mindful practices, while engineers and scientists push the boundaries of technology toward a more sustainable future. The interplay between various energy forms teaches us that although we cannot create or destroy energy, we can influence its path. Thus, the question emerges: How will you contribute to energy conservation in your daily life and beyond?