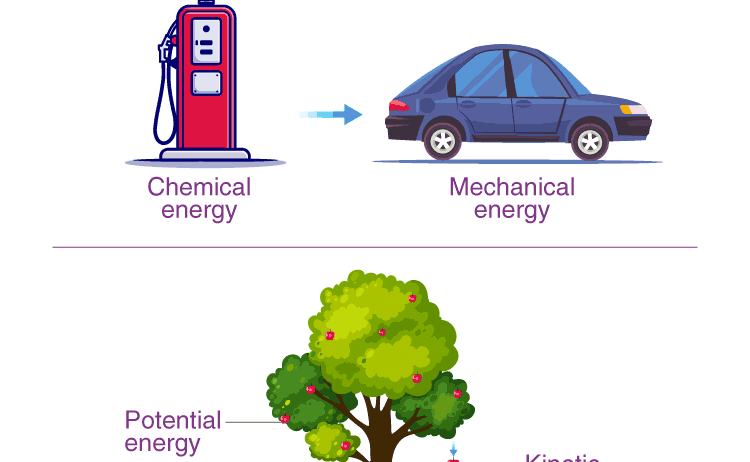
The Law of Conservation of Energy is not merely an abstract concept; it is a fundamental principle that governs the physical world. Often likened to a grand symphony, where every note plays a critical role in the overall harmony, the law articulates that energy cannot be created or destroyed, only transformed. This elegant notion has been sculpted through centuries of scientific inquiry, emerging as a cornerstone of physics that resonates across diverse disciplines.
The origins of the Law of Conservation of Energy can be traced back to antiquity, where early philosophers, such as Aristotle, pondered the nature of matter and motion. Aristotle theorized that objects possess innate qualities that could be altered but not annihilated. However, it wasn’t until the dawn of the Enlightenment, a period ripe with intellectual fervor, that a more empirical understanding began to coalesce. It was then that the stubborn tapestry of energy, woven into the fabric of nature, began to unravel its secrets.
One of the pivotal figures in the development of this law was Sir Isaac Newton. With his monumental work, “Philosophiæ Naturalis Principia Mathematica,” published in 1687, he laid the groundwork for classical mechanics. Newton’s laws of motion elucidated the relationship between force, mass, and acceleration, but the concept of energy remained nebulous. It wasn’t until the 19th century that the threads began to interlace, culminating in the detailed understanding of energy as we know it today.
The Industrial Revolution, a period characterized by rapid advancements in technology and engineering, acted as a crucible for the refinement of this principle. As steam engines roared to life and machinery touched the skies, scientists began to measure and manipulate energy in unprecedented ways. The relationships between heat, work, and energy were scrutinized meticulously. It was during this ferment that the term “energy” began to emerge in scientific vernacular, attributed largely to French physicist Gaspard-Gustave de Coriolis and later, German physicist Hermann von Helmholtz.
The works of these pioneers culminated in what is now referred to as the First Law of Thermodynamics. Published in the mid-19th century, it formalized the notion that the total energy in a closed system remains constant. Like a meticulous accountant, the law ensures that energy in all its forms—be it kinetic, potential, thermal, or electromagnetic—accounts for balance despite its myriad transformations.
Simultaneously, the discovery of mechanical energy conservation reverberated through academic circles. Physicists such as James Prescott Joule conducted groundbreaking experiments, revealing that heat is a form of energy. His famous experiment, where mechanical work was converted into heat through friction, demonstrated this interchangeability by showing that energy is lost only in one form and gained in another. This was a clarion call that would echo through the annals of science, advancing our understanding of energy transformations.
While the principles gained traction, the metaphysical implications of the law also stirred philosophical discourse. The idea that energy ebbs and flows—transforming yet indestructible—invoked contemplation on existence and the sustainability of life itself. The natural world appeared as a dynamic energy dance, choreographed by the laws of physics. This intrinsic relationship between energy transformation and conservation not only shapes our environment but also informs our responsibilities in its stewardship.
As the 20th century unfolded, the Law of Conservation of Energy found its place among the foundational tenets of modern physics. The advent of theories such as relativity and quantum mechanics brought new dimensions to our understanding, suggesting that mass and energy are interchangeable via Einstein’s famous equation, E=mc². This revelation rendered the law more profound, as it implied that even the most seemingly solid matter is but a manifestation of its energetic constituents.
The significance of the Law of Conservation of Energy has now permeated various domains, influencing fields ranging from environmental science to engineering. In the context of climate change and sustainability, understanding this law is imperative. It serves as a reminder that energy efficiency and conservation efforts are not merely beneficial, but essential in maintaining the delicate balance of our ecosystems.
In the modern era, as we grapple with existential crises such as global warming and depleting nonrenewable resources, the Law of Conservation of Energy emerges as a guiding light. It emphasizes the importance of transitioning towards renewable sources of energy—solar, wind, and hydroelectric—transforming the very nature of how we harness power. The metaphor of the planet as a “closed system,” where energies recycle within, calls us to adopt sustainable practices that minimize waste and encourage a harmonious coexistence with our environment.
In conclusion, the evolution of the Law of Conservation of Energy reflects humanity’s relentless quest for understanding the universe. From Aristotle’s musings to the empirical investigations of physicists, the journey has been extraordinary. This enduring principle not only elucidates the behaviors and interactions of various forms of energy within systems but also serves as a compelling reminder of our duties as custodians of the Earth. The harmony of energy, akin to a grand symphonic performance, continues to resonate, beckoning us to listen and respond with awareness and action.