The principle of conservation of energy is an intriguing and fundamental concept in the field of thermodynamics. Often heralded as the First Law of Thermodynamics, it provides a robust framework for understanding how energy operates within our universe. This law posits that energy cannot be created or destroyed; rather, it can only be transformed from one form to another. This idea not only reshapes our understanding of physical processes but also challenges our perceptions about energy use in everyday life. As we delve deeper, this thrilling exploration reveals both a scientific doctrine and a vital conduit for environmental advocacy.
To fully grasp the significance of the conservation of energy, one must first appreciate its historical context. The development of this concept traces back through the centuries, intersecting with the works of notable figures such as Galileo, Newton, and later, Joule. Their collective contributions paved the way for a paradigm shift in how energy was perceived. Prior to the establishment of this principle, many believed in a rather qualitative view of energy. The formulation of the First Law solidified a quantitative appreciation of energy, establishing the framework for modern physics and engineering.
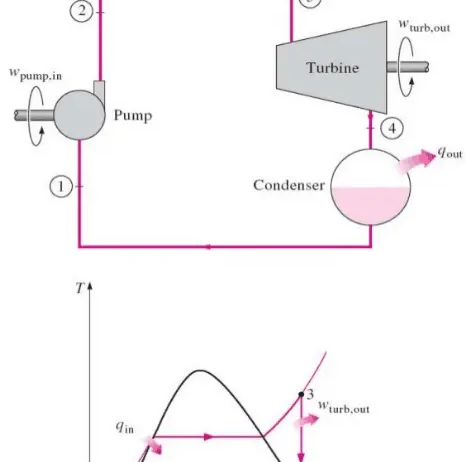
At its core, the First Law of Thermodynamics encapsulates the idea that the total energy of an isolated system remains constant. This law governs the behavior of everything, from the mundane workings of household appliances to the vast complexity of astronomical phenomena. It encompasses various forms of energy, including kinetic, potential, thermal, and chemical energy. The interplay among these forms showcases the transformative power of energy within systems, becoming particularly relevant when considering efficiency and conservation strategies.
One cannot ignore the implications of the conservation of energy on environmental science and sustainability. The acknowledgment that energy can only change form nudges society towards a more holistic understanding of resource management. For instance, the focus on renewable energy sources stems from the recognition that while fossil fuels are inherently finite, solar, wind, and hydroelectric systems continually regenerate. This knowledge is pivotal in inspiring a transition towards sustainable energy practices, empowering individuals and societies to minimize waste and optimize usage.
Furthermore, the conservation of energy serves as a cornerstone for innovations aimed at energy efficiency. By understanding how energy transforms, engineers and scientists have developed advanced technologies that capture and reallocate energy rather than letting it dissipate as waste. Such applications reside at the heart of modern engineering, influencing sectors including transportation, construction, and manufacturing. From our cars to our electric grids, every facet of modern life is intertwined with the principles of thermodynamics and energy conservation.
Nevertheless, the implications of the First Law extend beyond practical applications. They provoke a philosophical inquiry into our consumption habits and societal paradigms. In an age characterized by overconsumption, the understanding of energy conservation invites individuals to rethink their personal carbon footprints. Are we merely users of energy, or stewards of a precious resource? This line of questioning highlights the ethical implications of energy use, stimulating a broader dialogue on sustainability.
An interesting aspect of the conservation of energy is its applicability to the human experience. The energetic exchanges that occur within ecosystems and between organisms are both delicate and dynamic. In ecology, the concept of energy flow through food webs exemplifies this principle. Energy enters ecosystems through photosynthesis, traverses various trophic levels, and ultimately dissipates as entropy in the form of heat. Recognizing this ebb and flow of energy fosters a deeper understanding of biodiversity and ecosystem resilience, underscoring the necessity of energy conservation in maintaining ecological balance.
Moreover, the assertion that energy cannot be destroyed implies that what we perceive as waste is, in reality, a transformation of energy that has gone unutilized. This concept is pivotal in waste management and circular economy initiatives. By redefining waste as a resource, communities can adopt sustainable practices that repurpose spent energy, thereby reducing landfill burdens and diminishing environmental impacts. A shift towards circularity can yield unprecedented benefits, showcasing the practical application of the conservation of energy in societal frameworks.
As we explore various fields—from thermodynamics and ecology to philosophy and engineering—the omnipresence of the conservation of energy unfolds, urging us to question habitual consumption tendencies. It beckons us to adopt an expansive perspective that recognizes interconnectedness in the fabric of life and energy use. The implications of this principle stretch far beyond academic musings, providing a vital impetus for policy change and social awareness.
A comprehensive understanding of the conservation of energy encourages curiosity and innovation. It inspires us to develop novel solutions in energy technology and behavioral shifts toward resourcefulness. As we confront the challenges of climate change and environmental degradation, the First Law of Thermodynamics stands as a guiding star, illuminating the path to a sustainable future.
In conclusion, the conservation of energy is not merely a scientific tenet; it is a clarion call for transformation in attitudes and actions. By internalizing this principle, we can leverage our understanding of thermodynamics to forge a sustainable world where energy is revered rather than squandered. Thus, the First Law of Thermodynamics encapsulates not only a fundamental scientific truth but also a profound invitation for global citizens to partake in the stewardship of our collective energy resources.