Energy conservation is a fundamental principle of physics that underpins numerous natural processes. When exploring interactions between two objects, the question arises: Is energy truly conserved? This inquiry leads us to consider various scenarios where energy exchanges may occur, revealing the complexity and beauty of energy conservation in dynamics.
To begin, let’s define what we mean by energy. In physics, energy is often described as the ability to do work. It exists in various forms, including kinetic, potential, thermal, chemical, and more. When two objects interact, the energy within the system can transform from one form to another, raising the query of whether the total energy remains constant.
Imagine two billiard balls colliding on a pool table. When they strike each other, they exchange momentum and energy. The kinetic energy of the moving ball is transferred to the stationary ball, causing it to move away. This dynamic interaction illustrates a critical notion: while kinetic energy may seem to diminish for the moving ball, it is crucial to recognize that energy itself is not lost but rather redistributed within the system.
However, it is essential to delve deeper than the simplistic view of energy exchange. In elastic collisions, both momentum and kinetic energy are conserved. This means that the total energy before and after the interaction remains unchanged. But what happens in inelastic collisions, where objects may stick together or deform? In these cases, some kinetic energy is transformed into other forms, such as thermal energy or sound, suggesting that not all kinetic energy is retained. This transition prompts a vital distinction: while kinetic energy might not be conserved, the overall energy in the system remains conserved.
Consider a scenario in a more complex system, such as a roller coaster. As it ascends, potential energy accumulates due to its height. Upon descending, this potential energy transforms back into kinetic energy, causing the coaster to accelerate. Throughout this exhilarating ride, the law of energy conservation is not violated; the energy merely changes its form, showcasing the dance between kinetic and potential energy. Yet, there are energy losses, primarily due to friction and air resistance, converting mechanical energy into heat, demonstrating that while the total energy remains conserved, its usability may diminish. This raises another layer of complexity in our understanding.
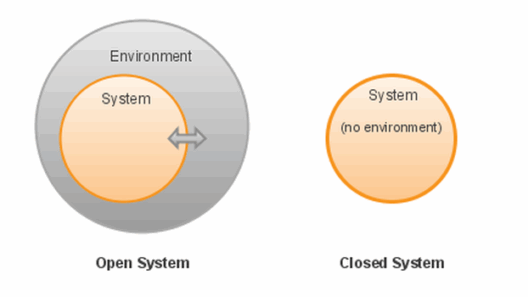
As we investigate systems further, consider two objects in a closed environment, such as two magnets interacting with each other. When they come close, the magnetic field exerts forces, leading to potential energy alterations. When they repel or attract, kinetic energy comes into play as they move. Throughout these interactions, energy transformations abound, emphasizing that while energy may seem to be absorbed or emitted, the total remains unchanged.
The intriguing concept of entropic energy emerges, guiding us to ponder whether energy is always available for work. In a closed system, as energy transforms, it tends to disperse. The Second Law of Thermodynamics states that entropy, or disorder, increases over time, affecting the energy’s capacity to perform work. In this way, you may notice that energy conservation is upheld; however, usable energy diminishes, complicating how we interpret conservation amidst dynamic interactions.
Moreover, the interaction between two objects is not always straightforward. One must account for the energy forms that may not be immediately visible, such as sound energy produced during a collision or thermal energy emitted when two surfaces rub against each other. When assessing whether energy is conserved, it is imperative to examine all forms of energy and the subtle exchanges occurring, illuminating a multifaceted conservation landscape.
This complexity beckons us to challenge our perceptions of energy conservation. Is energy truly conserved, or merely transformed? The answer lies in understanding that conservation is a matter of perspective. While energy maintains its totality in an isolated system, the challenge rests in identifying and accounting for all forms of energy and their respective transitions.
When addressing practical implications of energy conservation between two interacting objects, numerous questions arise. How can we harness energy efficiently to minimize losses during interactions? In industries such as manufacturing and transportation, embracing energy-efficient designs that consider friction and heat loss is crucial. This quest towards energy optimization opens avenues for sustainable practices, aligning with our overarching goals of environmental conservation.
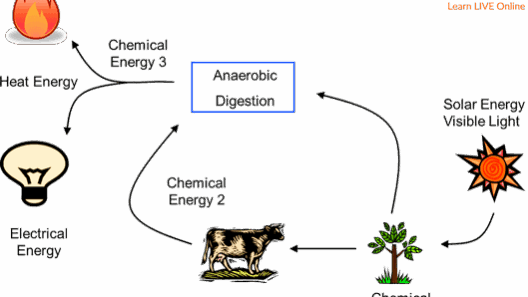
Additionally, examining energy conservation within biological systems offers insights into nature’s efficiencies. Take photosynthesis, for example, where plants convert solar energy into chemical energy. Here, energy repurposing exemplifies the ingenious energy transformations occurring within natural systems. It reiterates the importance of conserving energy, not merely in a physical sense but also in ecological contexts.
In conclusion, energy is conserved within interactions between two objects, but the manifestation of that conservation may vary dramatically based on the circumstances. While kinetic energy may not always remain constant during exchanges, the overall energy of a closed system remains invariant, subject to transformation across various forms. Recognizing and appreciating the subtleties of energy interactions can galvanize our efforts in energy conservation and inspire innovative approaches toward sustainability. Whether through scientific inquiry or everyday applications, understanding the principles of energy conservation can lead to transformative changes in how we engage with our environment.