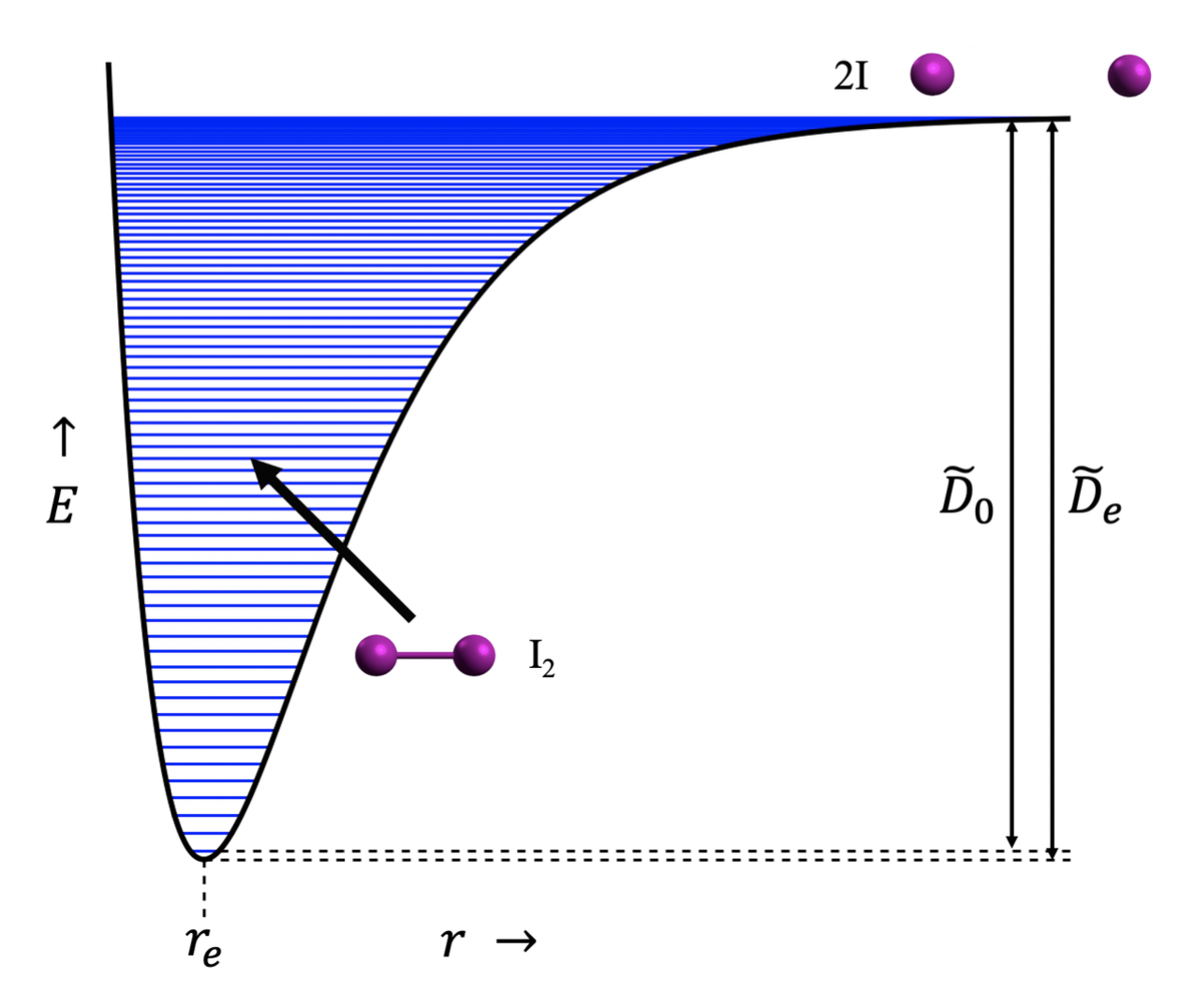
Energy conservation is a fundamental principle rooted in the laws of thermodynamics, heavily influencing both physical chemistry and environmental science. To fully appreciate how energy is conserved during a chemical reaction, one must dissect the processes of bond formation and cleavage while examining the overarching laws of energy transformation.
At the molecular level, a chemical reaction entails the breaking of existing bonds and the formation of new ones. This might seem to imply a straightforward exchange, yet the complexity of energy dynamics complicates the matter. When bonds between atoms are broken, energy must be supplied to overcome the attractive forces that hold the atoms together. The requisite energy for this disruption of bonds is referred to as bond dissociation energy. Conversely, forming new bonds releases energy, often termed bond formation energy. The interplay of these two energies is crucial in determining the overall energy change of a reaction.
The first law of thermodynamics states that energy cannot be created or destroyed, only transformed. This law, often summarized as the conservation of energy principle, is pertinent in understanding chemical reactions. In essence, the total energy contained in the reactants must equal the total energy of the products, although it may manifest in various forms. For instance, in an exothermic reaction, the system releases heat energy to the surroundings, while in an endothermic reaction, energy is absorbed from the surroundings.
When analyzing a chemical reaction, one must consider several nuanced components. These include the nature of the reactants, the energy required to initiate the reaction, and the enthalpy change associated with the transformation. The enthalpy change, denoted as ΔH, quantifies the total energy exchange during the reaction. A negative ΔH indicates an exothermic reaction, while a positive ΔH signifies an endothermic process. In both cases, the law of conservation of energy holds – energy is conserved, merely transitioning between forms.
Different types of energy play significant roles in predicting the behavior of chemical reactions. Chemical energy, found within the bonds of molecules, is released or absorbed during chemical transformations. Thermal energy, influenced by temperature, can accelerate molecular motion, thus affecting reaction rates and mechanisms. Additionally, light energy may initiate specific reactions, such as photosynthesis, highlighting the broad spectrum of energy interactions in chemistry.
One must also consider the concept of activation energy – the energy threshold necessary for a reaction to occur. This barrier must be overcome irrespective of whether the reaction is exothermic or endothermic. Catalysts may serve to lower the activation energy, enhancing the likelihood of successful collisions between reactants. As a result, the reaction can proceed more rapidly without violating the principle of energy conservation. Indeed, energy is transformed and conserved through alternative pathways when catalysts are involved.
In practical applications, the conservation of energy principle serves as a guideline for evaluating energy efficiency in chemical processes. Industries increasingly strive to maximize the efficacy of chemical reactions while minimizing energy waste. This encompasses optimizing reaction conditions, employing catalysts, and integrating sustainable practices. Such strategies are particularly vital in sectors heavily reliant on chemical reactions, such as pharmaceuticals, energy production, and materials science.
The emphasis on energy conservation extends beyond industrial practices to encompass broader environmental ramifications. Understanding energy dynamics within chemical reactions contributes to the development of sustainable technologies and practices. For instance, biofuels derived from biomass undergo intricate chemical transformations that hinge on energy conservation principles. By examining the energy flows within these processes, one can enhance the sustainability and efficiency of renewable energy sources.
Moreover, controversies surrounding energy conservation in chemical reactions often arise in specific areas, such as nuclear chemistry. In nuclear reactions, the associated energies stem from the nucleus rather than atomic bonds, challenging the traditional notions of chemical reaction energy dynamics. Although the principles of energy conservation remain applicable, the mechanisms diverge significantly. The debate between chemical and nuclear reactions serves as an intriguing contrast within the broader dialogue on energy conservation.
In conclusion, the question of whether energy is conserved during chemical reactions is resolutely affirmed by the first law of thermodynamics. While bonds are broken and new ones formed, the total energy remains constant, merely transitioning between various forms. A comprehensive understanding of this dynamic requires an exploration of the types of energy involved, the energy changes quantified through enthalpy, the barriers to reaction, and the implications for sustainable practices. Ultimately, the congruence of energy conservation with chemical transformations underlines the importance of integrating scientific principles into practical applications aimed at promoting environmental stewardship.