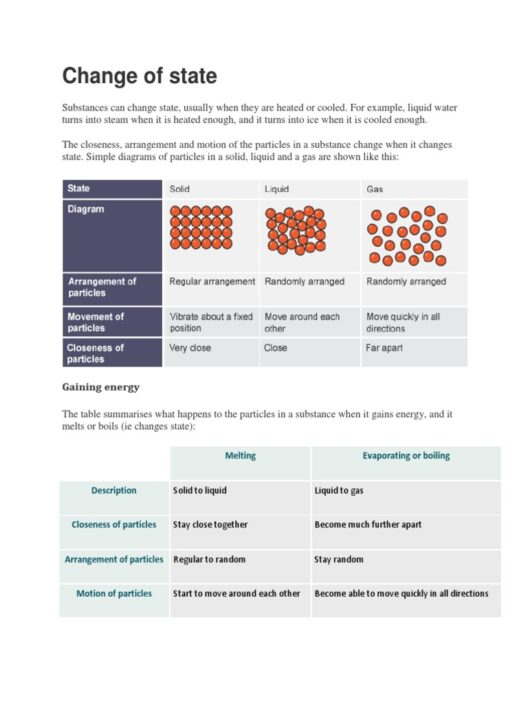
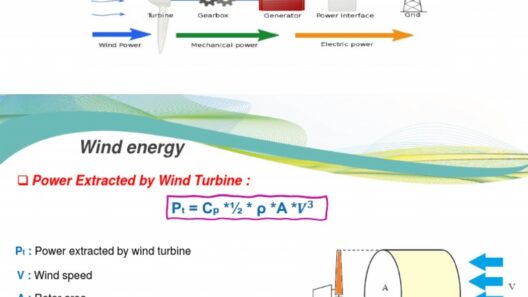
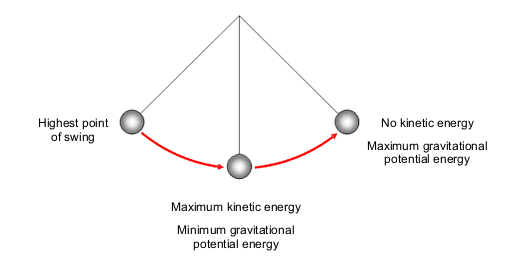
Energy conservation is a cornerstone of thermodynamic theory, which has profound implications for both scientific inquiry and practical energy management. The question, “Is energy conserved in an open system?” transcends basic theoretical discussions and delves into the intricacies of energy flows. An open system, by definition, can exchange both matter and energy with its surroundings, which poses a unique challenge to classical thermodynamic principles.
To understand energy conservation, let’s first delineate the fundamental concepts of an open system in thermodynamics. An open system allows for the transfer of energy through work and heat, along with the exchange of mass. This fluid exchange prompts the question: if energy can enter and exit freely, how can we assert that energy is conserved?
The First Law of Thermodynamics, often expressed as the principle of energy conservation, states that energy cannot be created or destroyed, only transformed from one form to another. In a closed system, where no mass or energy crosses the boundaries, this law holds true and straightforward. Energy transformations may occur, such as a heating process or mechanical work, but the total energy remains invariant.
However, as we transition to consider open systems, the boundaries become less clear. Here, energy influx and efflux must be meticulously accounted for. A matter of curiosity arises: can we track energy changes accurately in systems where energy continuously enters or leaves? For instance, consider a lake receiving sunlight, which drives photosynthetic processes in aquatic plants, while simultaneously losing heat to the atmosphere. The simultaneous influx and efflux of energy means that the energy balance is continually shifting. This leads us to ponder: how can we fairly assess conservation when energy in an open system is in constant flux?
To tackle this question, the concept of energy accounting comes into play. The principle of energy conservation still applies; however, it requires a nuanced approach. In an open system, we can adopt a boundary that encompasses all significant energy and mass exchanges. By applying the First Law, we can represent the change in internal energy as a function of heat exchanged and work done on or by the system. Therefore, the statement “energy is conserved” morphs into a more complicated schema: energy is conserved when all inputs and outputs are considered.
To illustrate this further, let us examine an ecosystem as a quintessential open system. An ecosystem undergoes continuous energy flow—sunlight enters, chemical energy is transformed through photosynthesis, and energy exits in various forms, such as heat during respiration. Even though energy is dissipated, it doesn’t violate the concept of conservation. Instead, the total energy in the system adjusts dynamically, always adhering to the principle of conservation when assessed over time.
This dynamic balancing act springs forth intriguing implications regarding sustainability and energy policies. If energy within open systems is indeed conserved, albeit in a more complex manner, it renders the pursuit of efficient energy usage as an imperative. How do we optimize this transfer and conversion to minimize waste? This question presides at the forefront of current environmental discussions, embodying both a challenge and an opportunity for innovation.
The application of energy conservation principles also intersects with the laws of entropy. The Second Law of Thermodynamics states that entropy, or disorder, in an isolated system tends to increase over time. In open systems, while energy is being conserved, entropy may still increase, which suggests that energy transformations are not entirely efficient. This interplay offers a further layer of complexity; as energy is transferred or transformed, the inevitable increase in entropy speaks to the inefficiencies inherent in energy usage. Hence, while we recognize energy conservation, we must also be cognizant of entropy’s role as an inevitable factor in the interplay of open systems.
Moreover, recognizing the limitations of energy conservation within these open systems fuels the dialogue surrounding renewable energy technologies. For instance, solar panels generate energy by harnessing sunlight—a quintessential example of energy exchange in an open system. The challenge emerges not from conservation but from maximizing energy capture relative to what is lost as heat or unused light. Therefore, our strategies hinge on enhancing efficiency, tapping into innovative materials or designs that can minimize energy loss during conversion.
Furthermore, economic systems could be reframed as open systems. Consider the energy costs associated with production and consumption in market dynamics. Just as ecosystems must account for energy inputs and outputs, so too must modern economies grapple with the sustainability of their energy use, factoring in renewable sources versus traditional fossil fuels. Can economic models integrate the principles of thermodynamics, accounting for energy conservation while also promoting sustainable practices? This remains a significant challenge for policymakers and economists alike.
In closing, the exploration of energy conservation within open systems invites rich discourse among environmentalists, scientists, and policymakers. Energy is indeed conserved, yet this essence varies significantly when we consider the complexities of open systems. By recognizing the fluidity of energy exchange and the overriding role of entropy, we can better navigate the challenges that lie in optimizing energy use for sustainability. Thus, the question standing before us is not merely whether energy is conserved, but how we can accentuate its efficiency in the multifaceted systems that define our environment and economies.