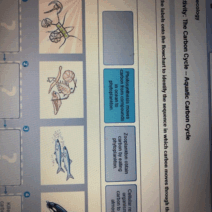
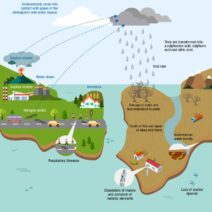
In the grand tapestry of the universe, the interplay of energy and matter is a critical theme woven through every chemical reaction. Energy conservation, a fundamental principle of physics, posits that energy cannot be created or destroyed; it can only transform from one form to another. This principle holds significant implications in the realm of chemical reactions, hinting at a nuanced understanding of energetic processes that underlie everyday phenomena. As we embark on this exploration, we will unpack the intricate relationship between energy and chemical reactions, shedding light on how energy conservation manifests in diverse contexts.
Chemical reactions abound in our daily lives, from the combustion of fuels that power our vehicles to the metabolic processes that sustain life within our bodies. At the heart of these reactions lies the concept of chemical potential energy, stored within the bonds of molecules. When a chemical reaction occurs, the bonds between atoms are broken and reformed, leading to the release or absorption of energy. Thus, understanding whether energy is conserved in these reactions necessitates a closer look at the mechanisms involved.
The law of conservation of energy asserts that in a closed system, the total energy remains constant. This is a crucial consideration in chemical reactions, which can be categorized into two broad types: exothermic and endothermic. Exothermic reactions, such as combustion, release energy to their surroundings as chemical bonds break and form new products. Think of a wood fire; as the wood burns, it releases heat and light, demonstrating energy transfer. Simultaneously, the internal energy of the reactants diminishes, as energy dissipates into the environment. In contrast, endothermic reactions, such as photosynthesis, necessitate an input of energy to proceed. In this case, plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. This process showcases the capture and temporary storage of solar energy, which drives the chemical transformation.
Upon closer examination, the interplay between energy conservation and chemical reactions reveals layers of complexity. The energy transformation can often be quantified using enthalpy, a thermodynamic quantity that reflects the heat content of a system at constant pressure. In exothermic reactions, the change in enthalpy (ΔH) is negative, indicating energy release, whereas it is positive for endothermic processes. Systems are designed to reach equilibrium, where the rates of the forward and reverse reactions are equal, contributing to a formulaic preservation of energy within the system.
This equilibrium showcases the dynamic nature of chemical reactions. Even in processes seemingly static, energy is in constant flux, exchanged between reactants and products at a molecular level. Furthermore, the conservation of energy extends beyond simple reactant and product transformations. It influences reaction kinetics, thermodynamics, and the intricate pathways that govern the performance of organic and inorganic compounds alike.
Let us delve into a striking instance illustrating energy conservation in chemical processes: the thermal decomposition of calcium carbonate (CaCO3) into calcium oxide (CaO) and carbon dioxide (CO2). This endothermic reaction requires the input of heat to proceed, highlighting the energy necessity for breaking the chemical bonds within calcium carbonate. Upon reaching the requisite temperature, thermal energy absorbed from the environment catalyzes the reaction, ultimately resulting in the generation of CaO and liberated CO2, thus reaffirming the principle of energy preservation. No energy is lost; it merely transitions from thermal to chemical potential energy.
Examining energy conservation through the lens of chemical reactions also sheds light on the entwined nature of energy systems across environmental and biological domains. Take, for instance, the pivotal role of energy in cellular respiration — a biochemical pathway by which living organisms convert glucose into usable energy through various chemical transformations. The ATP (adenosine triphosphate) produced during cellular respiration is a prime example of energy conservation. The energy harnessed during glucose catabolism is captured in the form of ATP, allowing organisms to utilize this energy in biochemical processes essential for life. Here, the cyclical nature of energy highlights its conservation throughout biological systems.
Moreover, understanding energy conservation in chemical reactions paves the way for innovations in sustainable practices and renewable energy technologies. In the quest to combat climate change, the development of energy storage systems and efficient chemical processes is paramount. Technologies such as batteries, solar panels, and fuel cells exemplify the practical applications of energy conservation principles. They embody the transformation of energy into storable forms, embodying the hope for a future where energy resources are managed sustainably.
As we stride into an era increasingly defined by energy challenges, it is critical to appreciate the extensive implications of energy conservation within chemical reactions. The delicate balance maintained through transformations, the interconnectedness between various energy forms, and their eventual applicability to sustainable practices holds promise for innovative solutions to current ecological challenges.
In conclusion, embracing a perspective rooted in the conservation of energy prompts intrigue and curiosity about the chemistry that permeates our world. Recognizing that energy is ever-present, continuously shifting forms, and fundamentally conserved within chemical reactions allows for deeper understanding. This knowledge not only enriches our comprehension of scientific principles but can also spur actions that reflect a commitment to environmental conservation and sustainable energy practices. The energy within chemical reactions is not merely a theoretical concept; it embodies a crucial narrative that influences everything from molecular interactions to global energy strategies. Thus, we find ourselves at a pivotal juncture where intellectual curiosity must translate into actionable change for a sustainable future.