Energy conservation is a principle that governs many physical and chemical processes, including redox reactions. The underlying question of whether energy is conserved during these reactions extends beyond mere observation; it dives into the intricate dance of electrons, chemical transformations, and the laws of thermodynamics. In order to comprehend this fascinating concept, one must explore various dimensions of redox reactions, encompassing their definitions, mechanisms, and implications for energy conservation.
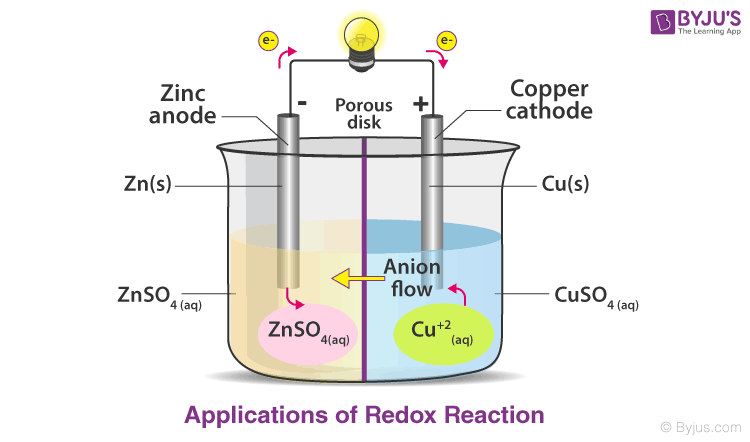
To begin, redox reactions, short for reduction-oxidation reactions, are chemical processes in which the oxidation states of the reactants undergo change. This alteration occurs as electrons are transferred from one substance to another. A reducing agent, which donates electrons, gets oxidized, while an oxidizing agent, which accepts electrons, gets reduced. The classic example is the reaction involving hydrogen and oxygen to form water: hydrogen is oxidized, and oxygen is reduced. The interplay between oxidation and reduction is fundamental to understanding energy shifts in these reactions.
At the core of energy conservation in redox reactions lies the principle of energy transfer. When bonds are formed or broken during these chemical reactions, energy is released or absorbed. This is consistent with the law of conservation of energy, which asserts that energy cannot be created or destroyed, only transformed from one form to another. In the case of redox reactions, the energy stored in chemical bonds is often converted into kinetic energy, thermal energy, or electrical energy. Therefore, while the energy appears to change form, it is not lost; rather, it is conserved through transformation.
Exploring the thermodynamics of redox reactions elucidates the conditions under which energy transformations occur. Gibbs free energy, a critical thermodynamic function, determines the spontaneity of a reaction. Negative Gibbs free energy signifies a spontaneous reaction, during which energy is released. For example, in the rusting of iron, iron oxidizes to form iron oxide, releasing energy in the process. This release can power various biological or industrial processes, further illustrating the conservation of energy as it transitions from chemical form to usable forms of energy.
Moreover, redox reactions are pivotal in biological systems. Cellular respiration, for instance, is a quintessential redox process wherein glucose is oxidized to carbon dioxide, and oxygen is reduced to water. The energy released during this conversion is captured in the form of ATP (adenosine triphosphate), the energy currency of the cell. Here, energy conservation manifests as the body transforms the potential energy in glucose into a format that sustains cellular functions. Thus, redox reactions not only highlight the conservation of energy but also its indispensable role in sustaining life.
Yet, the notion of conservation in redox reactions transcends mere energy transfer. It beckons a multifaceted understanding of efficiency, environmental impact, and technological applications. In the realm of energy production, redox reactions underpin batteries and fuel cells, devices that convert chemical energy into electrical energy. In these systems, the efficiency of energy transformation determines their viability and environmental footprint. The advancements in battery technology, particularly those using redox reactions, are valuable as societies transition towards renewable energy sources, thereby aligning energy conservation with sustainability.
One might ask, how do inefficiencies in redox reactions manifest? The answer lies in the thermodynamic constraints and kinetic barriers that impede the complete conversion of energy. For instance, in combustion reactions, such as burning fossil fuels, redox reactions yield energy but also produce pollutants and greenhouse gases. These outcomes pose a dual challenge: energy conservation must be pursued alongside environmental conservation. Innovations in catalytic processes aim to enhance the efficiency of redox reactions, reducing waste and harnessing energy more effectively.
Furthermore, the educational aspect of redox reactions merits consideration. By delving into these reactions, students and researchers alike gain insights into fundamental principles of chemistry, physics, and environmental science. Many educational curricula emphasize the importance of understanding redox reactions due to their ubiquitous presence in various fields, from industrial chemistry to biochemistry. Cultivating a deeper comprehension of these processes empowers the next generation to tackle the critical challenges of energy conservation and resource management.
As one examines redox reactions through the lens of energy conservation, a symbiotic relationship emerges: the conservation of energy is not merely a passive observation but an active principle that shapes our world. The underlying mechanisms of electron transfer, bond changes, and energy transformations offer a compelling narrative. Through cellular processes, technological applications, and environmental considerations, the importance of redox reactions in the context of energy conservation becomes unequivocal.
In conclusion, the inquiry into whether energy is conserved in redox reactions allows for a rich exploration of chemistry and environmental science. By understanding the principles of electron transfer and thermodynamics, one can appreciate the complexity and beauty of these reactions. As societies strive for sustainability, the pursuit of efficient and innovative redox processes will play a pivotal role in harmonizing energy need with ecological responsibility. Embracing this knowledge equips individuals not only with scientific insight but also with the understanding that energy conservation is central to the ethos of environmental stewardship.