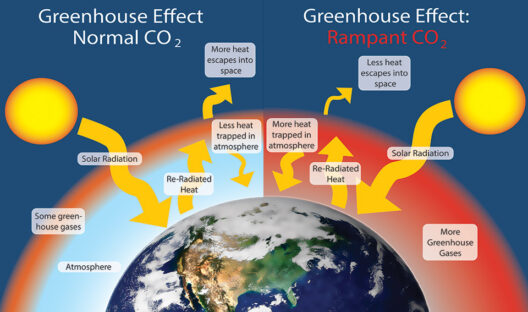
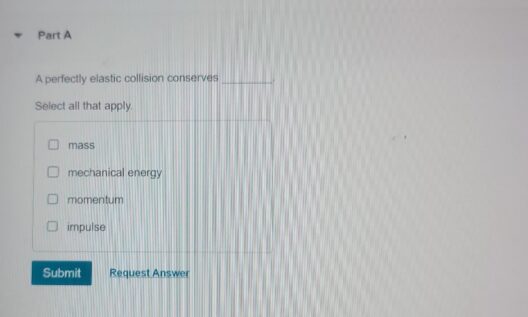
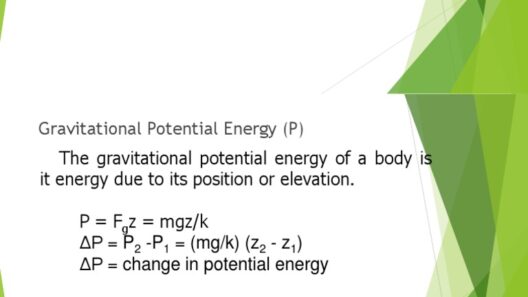
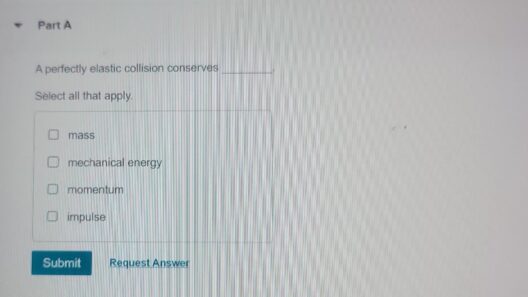
In the grand theater of the universe, where atoms perform their intricate dance, the principles of conservation of matter and energy emerge as fundamental actors. These principles dictate that during a chemical reaction, the components involved undergo transformation rather than annihilation. The interplay of matter and energy is akin to a grand symphony where each note represents an atom, and every crescendo symbolizes a bond being forged or broken. Understanding this symphony is essential to grasp the essence of chemical reactions.
At the heart of the matter is the law of conservation of mass, a principle that states matter cannot be created or destroyed in an isolated system. This tenet was elegantly articulated by Antoine Lavoisier in the late 18th century when he observed that the mass of the reactants equals the mass of the products. Imagine a baker mixing flour, sugar, and eggs to create a cake. Once baked, the components have transformed in appearance and structure, yet the total mass remains unchanged. This metaphor extends to chemical reactions, where reactants metamorphose into products without deviation in overall mass, affirming the permanence of matter.
The transformation process, however, is not a mere reallocation of physical substances; it encapsulates the very essence of energy exchange. As matter transitions through different states, energy withers away, transforms, and is rekindled. This phenomenon is expressed through the law of conservation of energy, which posits that energy can neither be created nor destroyed; it can only be converted from one form to another. Herein lies another captivating metaphor: consider energy as a flowing river. In its journey, it carves through materials, shapes landscapes, and nourishes life, yet the water itself remains ever-present, merely changing its form as it moves through the ecosystem of a chemical reaction.
During a chemical reaction, reactants possess potential energy, often encapsulated in the bonds that bind them. As these bonds are broken, energy is released or absorbed, depending on the nature of the reaction. Exothermic reactions, such as combustion, exemplify energy release. These reactions ignite a clamor of energy, lighting up the surrounding environment while leaving behind products that are less energetic. In contrast, endothermic reactions, like photosynthesis, demonstrate the absorption of energy. Here, energy is captured from sunlight, culminating in the transformation of carbon dioxide and water into glucose, a process vital for sustaining life on Earth.
This energetic interplay brings forth a delightful complexity. The dynamic exchange during a reaction mirrors the rhythm of life itself—ever-changing yet anchored in foundational principles. As bonds break and new connections form, the system does not lose its essence; rather, it evolves, presenting new compounds with unique properties. A deeper understanding of this process reveals that energy plays a crucial role in dictating the direction and spontaneity of reactions—much like the winds guiding a ship across the ocean.
To further appreciate the conservation principles in chemical reactions, one must also acknowledge the pivotal role of catalysts. Catalysts are the unsung heroes of the chemical world, offering an alternative pathway that lowers the energy barrier for reactions to occur. They expedite the transformation without being consumed in the process, demonstrating a unique twist on conservation. While they hasten reactions, catalysts do not alter the overall energy balance; they facilitate the same conservation laws, acting as adept guides in the vast expanse of molecular dynamics.
Visualizing chemical reactions through the lens of conservation can propel our understanding beyond mere equations. Imagine a bustling marketplace where vendors (reactants) exchange goods (atoms and energy), creating new products that characterize their trade. This marketplace exists within a closed system, ensuring that though forms may change and goods are reallocated, the inventory remains consistent. Understanding this marketplace allows scientists to predict outcomes, derive new compounds, and innovate methodologies across various disciplines, from pharmaceutical development to sustainable energy solutions.
However, the principles of conservation are not limited to the microscopic realm. They resonate with broader environmental implications. Energy conservation, for instance, is crucial when addressing global challenges. The finite nature of fossil fuels highlights the urgent need to adopt sustainable practices, harnessing renewable resources effectively. By acknowledging that energy cannot just vanish, yet can be transformed, mankind can optimize resource usage, minimizing waste and maximizing efficiency.
The elegance of chemical reactions, grounded in the conservation of matter and energy, illustrates a larger narrative—one of balance and unity within the universe. This dance of particles serves as a reminder that transformation is not synonymous with loss; rather, it embodies continuous evolution. As we endeavor to navigate the complexities of our environment, understanding these foundational principles can inspire innovative solutions that harness nature’s design, promoting sustainability while embracing progress.
In conclusion, the conservation of matter and energy during chemical reactions is a profound concept that underlines the principles governing our universe. Each reaction serves as a testament to the stability of mass and the versatility of energy, underscoring the interconnectedness of all things. By embracing these principles, we enhance our understanding of both the microscopic and macroscopic systems that shape our world, anchoring our efforts in a sustainable future built upon these timeless truths.