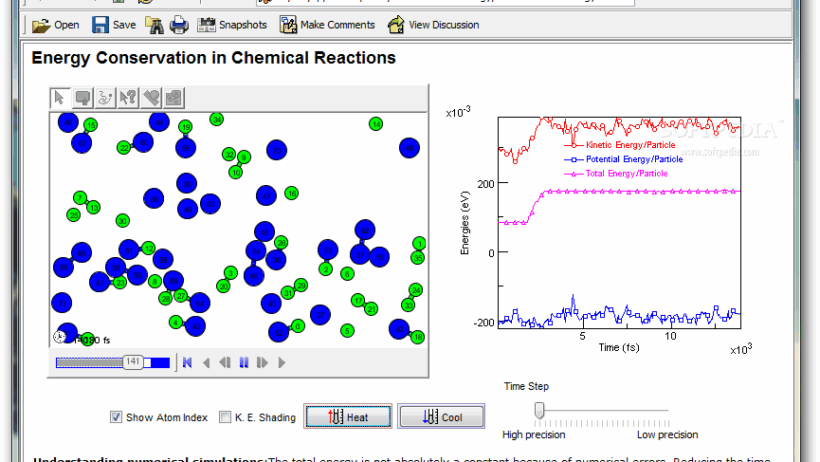
Conservation of energy is a fundamental principle in various scientific disciplines, including chemistry. This concept dictates that energy cannot be created or destroyed; instead, it merely changes forms. In chemical reactions, energy is meticulously conserved as bonds break and form, making it essential to explore the nuances of this principle in the context of chemistry.
Defining Energy Conservation
The principle of conservation of energy can be succinctly stated: the total energy of an isolated system remains constant. In chemical systems, this involves both kinetic and potential energy. Kinetic energy pertains to the motion of particles, whereas potential energy relates to the stored energy within bonds between atoms and molecules. Understanding these forms of energy is pivotal for comprehending the behavior of chemical reactions.
Types of Chemical Reactions
Chemical reactions are diverse and can be classified into various categories, each showcasing unique energy transitions. Exothermic reactions release energy into their surroundings, often in the form of heat, while endothermic reactions absorb energy. A classic example of an exothermic reaction is combustion, wherein fuels react with oxygen, releasing heat. In contrast, photosynthesis epitomizes an endothermic process; plants absorb sunlight, converting it into chemical energy stored in glucose.
The Role of Activation Energy
Activation energy is also a critical component in the conservation of energy within chemical reactions. It is the minimum energy required to initiate a reaction. Even in exothermic reactions, the system requires an initial input of energy to overcome the energy barrier, allowing reactants to transform into products. Understanding activation energy is essential for manipulating reaction rates and conditions, revealing yet another layer of energy conservation in practice.
Thermodynamics: The Energy Framework
Thermodynamics, the study of energy transfer, provides a framework for analyzing chemical systems. The first law of thermodynamics, which enshrines energy conservation, is paramount in predicting the energy changes associated with reactions. This law dictates that any heat produced or consumed in a reaction must correspond to changes in the internal energy of the system, as well as work done on or by the system. Applying these concepts enables chemists to quantify the energy changes that accompany chemical processes.
Enthalpy and Entropy
In the realm of thermodynamics, the concepts of enthalpy and entropy are integral to understanding energy conservation. Enthalpy (ΔH) is the measure of total energy within a system, aiding in the description of heat changes during reactions. On the other hand, entropy (ΔS) represents the degree of disorder within a system. The interplay between enthalpy and entropy determines reaction spontaneity and direction, underscoring the dynamic nature of energy conservation during chemical transformations.
Kinetics and Catalysis
The field of kinetics studies the rates of chemical reactions and the factors influencing these rates. The conservation of energy is critical here as well. Catalysts, substances that accelerate reactions without being consumed, operate by providing alternative pathways with lower activation energies. This alteration allows more reactant molecules to possess the requisite energy for a reaction, thereby facilitating the conservation of energy while enhancing the efficiency of the process.
Practical Implications of Energy Conservation
The implications of the conservation of energy extend beyond theoretical frameworks into practical applications. Industries rely on the principles of energy conservation for efficient process design and optimization. For instance, in industrial synthesis, careful control of thermal conditions ensures that energy is conserved, minimizing costs and maximizing yield. Furthermore, in the context of green chemistry, energy-efficient reactions minimize environmental impact, demonstrating the substantial role of energy conservation in sustainable practices.
Everyday Examples of Energy Conservation
Energy conservation principles are also observable in everyday life. The food we consume undergoes metabolic reactions that follow these laws; the energy stored in food is transformed into usable energy for bodily functions. Similarly, the combustion of fossil fuels in vehicles illustrates the conversion and conservation of energy, albeit with significant environmental drawbacks. Understanding these processes helps highlight the crucial need for sustainable practices and renewable energy sources to mitigate environmental impacts.
Conclusion
In summary, the conservation of energy in chemistry is an intricate interplay of principles and reactions that dictate how energy is transformed rather than created or destroyed. By exploring a multitude of reactions ranging from exothermic to endothermic processes, and understanding concepts such as activation energy, thermodynamics, kinetics, and catalysis, one gains insightful perspectives on how energy is conserved throughout these systems. Moreover, recognizing the practical implications of this principle can lead to improved efficiencies and enhanced sustainability in chemical practices. Ultimately, comprehending the science behind the conservation of energy in chemistry serves as a vital foundation for addressing contemporary environmental challenges while promoting sustainability.