The phrase “energy is conserved” refers to one of the fundamental principles in physics known as the Law of Conservation of Energy. This principle asserts that energy cannot be created or destroyed; it can only change forms or transfer from one object to another. Understanding this law is paramount in various fields, including physics, engineering, and environmental science, and its implications are vast. This discussion delves deeply into what it means for energy to be conserved, the various forms of energy, practical examples of the law in action, and its significance in contemporary environmental conservation efforts.
At its core, the Law of Conservation of Energy states that the total energy within an isolated system remains constant. Energy may shift from one type to another—such as kinetic energy transforming into potential energy—but the aggregate amount stays the same. This pivotal concept is grounded in empirical observations and mathematical formulations. The first law of thermodynamics is a formalization of this idea, stating that the total energy in a closed system is invariant over time. As such, every time work is done or heat is transferred, the energy within the system is merely redistributing.
Energy exists in multiple forms, each with unique characteristics and applications. The primary types include:
- Kinetic Energy: The energy of motion. Any object in motion possesses kinetic energy, which increases with speed. For instance, a rolling ball, a flowing river, or the vehicular traffic on a highway all exhibit kinetic energy.
- Potential Energy: Stored energy based on an object’s position or state. Common examples include gravitational potential energy, where an object held at a height has the potential to convert this energy to kinetic energy upon falling. Similarly, elastic potential energy is stored in compressed springs or stretched rubber bands.
- Thermal Energy: Often seen in the form of heat, thermal energy arises from the movement of particles within a substance. Higher temperatures correlate with greater particle motion, consequently increasing thermal energy.
- Chemical Energy: This type pertains to the energy stored within chemical bonds. It is released during chemical reactions, as seen in metabolizing food or the combustion of fossil fuels.
- Nuclear Energy: Energy released during nuclear reactions, either through fission (splitting atoms) or fusion (combining atoms). This form of energy has significant practical applications in power generation.
- Electrical Energy: The energy derived from the flow of electric charge. Electrical energy is a crucial form used in homes and industries alike.
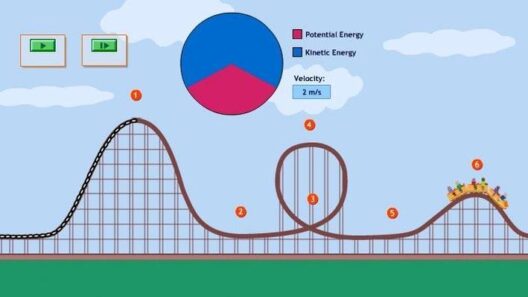
Recognizing different energy types aids in grasping how energy conservation operates within both natural and engineered systems. To illustrate, consider a classic example involving a pendulum. At its highest point, the pendulum possesses maximum potential energy, which converts to kinetic energy as it descends. At its lowest point, kinetic energy peaks while potential energy nears zero. As the pendulum swings back up, energy conversion occurs again. Throughout this cycle, the total energy remains constant, despite fluctuations between kinetic and potential forms.
In the realm of environmental science, comprehending energy conservation is essential for mitigating ecological impacts. The principle underlies many energy-efficient technologies and renewable energy systems. For example, understanding energy conservation has spurred advancements in wind, solar, and hydroelectric power—each method harnessing and transforming natural energy sources into usable forms without depleting them.
Furthermore, energy conservation plays a pivotal role in climate change mitigation. As fossil fuel reserves dwindle and greenhouse gas emissions reach alarming levels, emphasis on sustainable energy practices becomes increasingly urgent. Renewable resources are not only infinite but also pivotal in adhering to the conservation principle. Solar panels, for instance, convert sunlight into electrical energy efficiently; solar energy exists abundantly, perpetually available for collection.
Another significant implication of the conservation of energy is in energy auditing—a process that enables us to evaluate and reduce energy usage in various settings, from households to organizations. This evaluation assesses energy inflow and outflow, identifying inefficiencies and areas for improvement. When individuals and businesses adopt energy-efficient practices, such as utilizing LED lighting, optimizing heating systems, or employing smart technology, they contribute directly to conservation efforts while lowering overheads.
Despite its prevalence and applications, misconceptions about energy conservation often arise. One common misunderstanding is the idea that energy can be created or destroyed through various actions or technological methods. In reality, energy merely transitions between states or systems. For example, when driving a vehicle, fuel undergoes combustion, releasing energy to propel the car forward; while energy appears to be consumed, it is actually transformed from chemical potential to kinetic energy, manifesting an essential adherence to the conservation law.
In summary, the notion that “energy is conserved” encapsulates a foundational truth of both physics and our environmental responsibilities. It underscores the inevitable transformations energy undergoes and invites stakeholders across all sectors to appreciate the importance of harnessing and preserving energy efficiently. By fostering a deeper understanding of energy conservation, one can advocate for practices that conserve not only energy but also our planet’s vital resources, paving the way for a more sustainable future. As we navigate an increasingly energy-dependent world, the principles of energy conservation will remain pivotal in shaping our environmental narratives.